This thread's been a long time coming. There have been several discussions about light therapy, so I figured it would be best to have a thread focused on it. From what I understand, the terms photobiomodulation and low level laser therapy can be used interchangeably. They essentially both refer to the use of specific wavelengths of light as a clinical tool to help heal and reverse disease. Light has effects both on the surface of the skin and can penetrate deeply into the tissues. It stimulates mitochondrial energy production, cell protection and detoxification.
In my opinion the most interesting effects of light seem to be its interaction with biological water, whereby it induces water structuring which: possibly provides activation energy for chemical reactions to take place, is responsible for the flow of lymph, blood and other fluid, and may provide a physical/electrical barrier to prevent unwanted solutes and ions from entering the cell.
Nonetheless, here is a good introductory article on the topic of red light therapy:
In my opinion the most interesting effects of light seem to be its interaction with biological water, whereby it induces water structuring which: possibly provides activation energy for chemical reactions to take place, is responsible for the flow of lymph, blood and other fluid, and may provide a physical/electrical barrier to prevent unwanted solutes and ions from entering the cell.
Nonetheless, here is a good introductory article on the topic of red light therapy:
The Therapeutic Effects of Red and Near-Infrared Light (2015)
"Penetrating red light is possibly the fundamental anti-stress factor for all organisms. The chronic deficiency of such light is, I think, the best explanation for the deterioration which occurs with aging." - Raymond Peat
1. Preface
About two years ago, I spent a lot time reading Ray Peat's articles, trying to make sense of his various ideas regarding health. In many of his articles, Peat wrote that red light is healthy and even crucial for well-being, because it can activate mitochondrial respiration.
For example, in his article Aging Eyes, Infant Eyes, and Excitable Tissues (2006), he wrote:
"Old observations such as Warburg’s, that visible light can restore the activity of the 'respiratory pigments,' showed without doubt that visible light is biochemically active. By the 1960s, several studies had been published showing the inhibition of respiratory enzymes by blue light, and their activation by red light."
Peat didn't give many references to justify his claims, but after doing some searches on PubMed, I realized that there are literally thousands of papers supporting his views.
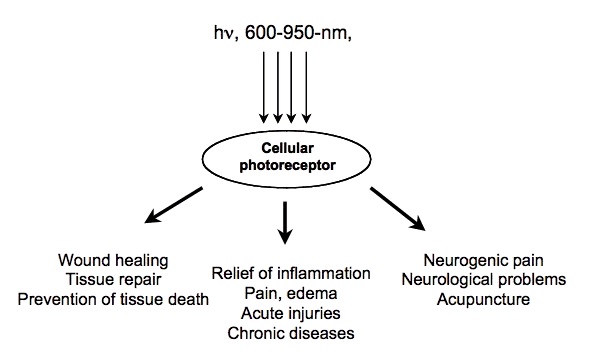
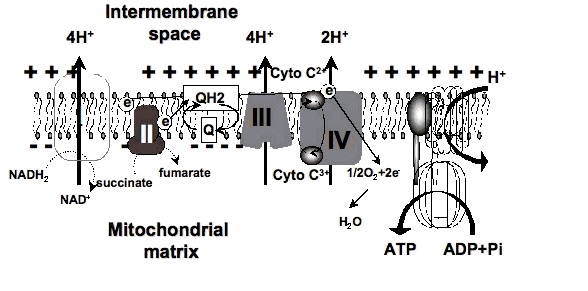
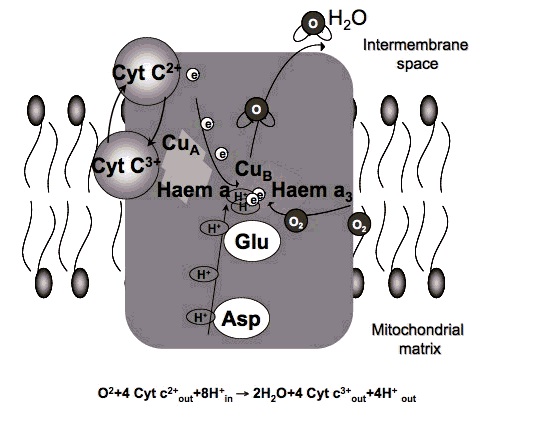
2. How could red light improve metabolism?
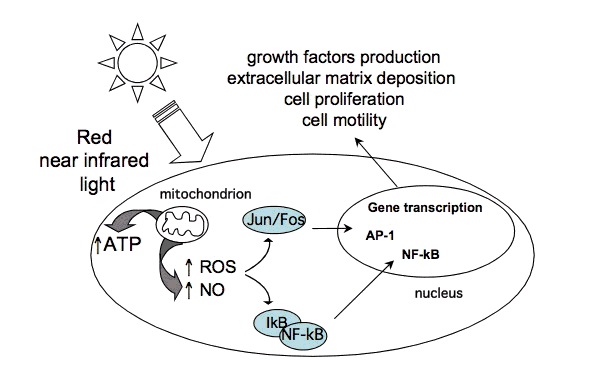
Certain wavelengths of electromagnetic radiation directly increase energy (ATP) in the tissues, and the activation of cytochrome c oxidase (Cox), the mitochondrial respiratory enzyme discovered by Nobel laureate Otto Warburg, seems to be the main mechanism.[1-6] On the molecular level, red light seemingly causes the photodissociation of nitric oxide (NO). When the cells are stressed, cells can produce NO which can bind to Cox, inhibiting its ability to bind oxygen.[2,7-11]
The following citations are from a news article on Nature (2006):
"Recent findings suggest that the enzyme [Warburg] identified, cytochrome oxidase, is a key player in a new understanding of how the cell's energy metabolism affects health and disease. And surprisingly they show that light has a profound effect on how the enzyme works — and could even be used to treat degenerative disease." [...]
"According to cell biologist Salvador Moncada of University College London, evolution really has crafted cytochrome oxidase to bind not only oxygen but also NO. 'One effect of slowing respiration in some locations is to divert oxygen elsewhere in cells and tissues,' he says. This prevents oxygen levels sinking dangerously low." [...]
"We have shown that light can indeed reverse the inhibition caused by NO binding to cytochrome oxidase, both in isolated mitochondria and in whole cells," says biochemist Guy Brown, at the University of Cambridge, UK. "And what's more, we found that light can protect cells against NO-induced cell death." [11]
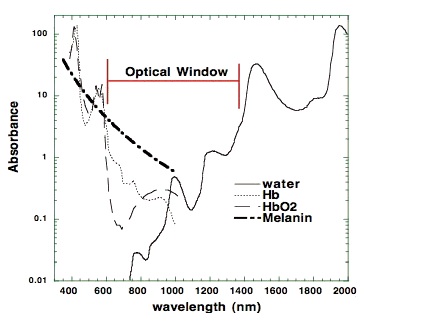
The most relevant wavelengths seem to be 600-1070nm --- in other words, red light and the penetrating shorter wavelengths of near-infrared radiation (NIR).[1,2]Our eyes are able to see 400-700nm radiation (blue, green and red light). Therapeutic effects come from 600-1070nm radiation (red and near-infrared light). Within this range, some wavelengths seem to have better effects than others. For example, in one study, 665nm and 810nm rays were beneficial, while 730nm and 980nm were not.[12]
There are important differences in the penetrating power of the different wavelengths. Visible red light doesn't penetrate tissue very well, but near-infrared does that quite well. If near-infrared is directed to the skin, the power seems to decrease 1000-fold by every 2-3 centimetres. However, a worm study shows that even those very small doses seem to have an effect on the cellular level.[13-15] Later in this article, I will also show that light apparently also has an systemic effect, which probably spreads through the circulation to the whole body. When it comes to Peat's claims about blue light, high intensities can inhibit the same enzyme (Cox), and this can lead to retinal damage and other problems.[11,16]
3. The health effects of red light and near-infrared radiation: The research history
John H. Kellogg's book (1910) is freely available on Archive.org website.
John Kellogg and incandescent lamp therapy (1910): The therapeutic use of red light is not a new phenomenon. The very earliest reports on the topic have been published in the 19th century, the most well-known article being The Red Light Treatment of Small-Pox (1895) by Niels Finsen, who also got the 1903 Nobel Prize in medicine, for his research regarding the health effects of light (especially ultraviolet radiation).[17]
In 1910, John Harvey Kellogg published his 200-page book Light Therapeutics, which included a large amount of information about the therapeutic usefulness of light therapy with incandescent light bulbs and arc lights. According to his book, light therapy can be effectively used for diabetes, obesity, chronic fatigue, insomnia, baldness, cachexia and many other health problems.[18] Similar therapeutic usage was also reported in Margaret A. Cleaves' book Light energy, its physics, physiological action and therapeutic applications (1904) and Leopold Freund's book Elements of general radio-therapy for practitioners (1904).
In the next decades after Kellogg's, Cleaves' and Freund's books, the information was apparently forgotten. However, some elderly people have told me that in their youth, some doctors used to recommend infrared lights for some therapeutic purposes (such as pain after dental operations).
The invention of laser light (1960-): In the beginning of 60's, the first red-light emitting lasers were invented. A couple of years after that, Endre Mester from Hungary reported that red laser light could improve hair growth on mice. Quickly after that it was also found that red laser light could accelerate wound healing in animals - and in '70s the first human trials were being done.[2] (At the same time, Marta Fenyo started focusing on polarized light therapy after working with Mester.)
In the '80s and '90s most of the research was conducted in the Soviet Union. There was many kinds of effects studied. For example, some studies reported protective effect against x-rays and other studies focused on heart disease.[19-22] Modern study of light/LLLT: In the Western countries, research on red light didn't really start before the 21th century, and most of the interesting studies have been released within the last five years (2010-2015). A dramatic amount of clinical research is being conducted nowadays: In 2014, more than 400 PubMed titles on the topic were published.
In most of the studies on red light or near-infrared therapy, a low-power laser device (output often less than 100mW) has been used, therefore the widely used abbreviation LLLT (low-level laser therapy).Many studies have also been conducted with LED's or other light sources. One can find also relevant studies by using keywords such as narrow-band light therapy, phototherapy, photobiomodulation, photobiostimulation, low-level light therapy, laser acupuncture, water-filtered infrared-A, near-infrared light, visible light, polarized-light therapy, or light-emitting diode irradiation. Despite different ways of generating the light, the wavelength used in the studies always fits between 600 and 1070 nanometres, because that is the effective range.
The studies have most often been conducted using a monochromatic light source (eg. 630, 633, 660, 670, 780, 808, 810, 890, 904, 940, 1064 or 1072nm). The parameters such as light dose, power output, pulsing or diameter of the light ray usually varies in the different studies because different devices are being used. There is no general consensus on which parameter is the most reliable, but many have yielded positive results.
4. The health effects of red light and near-infrared radiation: The extent of research
Today, PubMed keyword LLLT returns more than 4000 scientific papers. Hundreds of clinical trials have been conducted, and dozens of systematic reviews can also be found.
Here is a list of some health issues that have been reported to improve by light therapy (usually low-level laser). In some cases, the effects are still disputed, while other results are confirmed by meta-analyses.
Photobiomodulation indications (human studies):
Achilles tendinitis
Muscle pain
Acne
Neuropathic foot ulcer
Age-related macular degeneration
Neck pain
Aphthous ulcer
Nipple pain
Bell’s palsy
Oral mucositis
Body contouring
Orthodontic pain
Bone fractures (hand)
Osteoarthritis
Burn scars
Postherpetic neuralgia
Carpal tunnel syndrome
Pressure ulcer
Chronic joint disorders
Radiation dermatitis
Dentin hypersensitivity
Raynaud’s phenomenon
Depression
Restenosis
Erythema
Rheumatoid arthritis
Fibromyalgia
Shoulder tendinopathy
Frozen shoulder
Skin ageing
Hair loss
Sternotomy (recovery)
Hand-foot-and-mouth disease
Stroke
Herpes labialis
Temporomandibular disorders
Hypothyroidism
Tennis elbow
Low back pain
Venous leg ulcer
Lymphedema
Wound healing
Muscle growth
Xerostomia
Many animal studies have also been conducted:
Achilles tendinitis
Laryngitis
Acne
Liver cirrhosis
Acute pain
Liver regeneration
Adipose tissue inflammation
Lung injury
Age-related macular degeneration
Muscle injury
Allergic asthma
Muscle loss (sarcopenia)
Alzheimer’s disease
Nerve injury
Arthritis
Osteomyelitis
Atherosclerosis
Methanol toxicity (eyes)
Auditory neuropathy
Multiple sclerosis
Bone graft incorporation
Myocardial infarct
Bone fracture
Myonecrosis
Burns
Neuropathic pain
Cancer
Opiate addiction
Colitis
Oral mucositis
COPD
Osteoarthritis
Dentin regeneration
Osteoporosis
Depression
Periodontitis
Diabetes (eyes)
Parkinson’s disease
Diabetes (kidney)
Pressure ulcer
Endophthalmitis
Radiation damage
Exercise performance
Rheumatoid arthritis
Hair loss
Spinal cord injury
Hearing loss
Stroke
Heart failure
Temporomandibular inflammation
Hemarthrosis
Thrombocytopenia
Hyperalgesia
Tinnitus
Hypertension
Traumatic brain injury
Kidney fibrosis
Wound infection
Kidney injury
5. The health effects of red light and near-infrared radiation: A few examples of the clinical study results
5.1. Age-related macular degeneration
German clinicians conducted a retrospective analysis of 200 elderly subjects with age-related macular degeneration (most of them also had cataracts). The subjects were treated using a LLLT device emitting near-infrared light (780nm). The light was targeted to their eyes, through the conjunctiva.[65] The subjects were treated four times during two weeks. Placebo group was given a mock treatment. In the LLLT group, the visual acuity was improved in 95% of the subjects. Most of them were able to see a few rows lower on the Snellen chart. The improved vision was maintained for 3-36 months after treatment. LLLT also appeared to improve edema, bleeding, metamorphosia, scotoma and dyschromatopsia in some patients.
5.2. Knee Osteoarthritis
Hungarian researchers studied the use of near-infrared LLLT in knee osteoarthritis patients, in a double-blinded placebo controlled trial (830nm).[73] Intervention group got infrared treatment on their affected joint twice a week, over a period of four weeks. The placebo group got a similar treatment of 100-fold lower intensity.
In the intervention group, the pain scores were (on a scale from 1 to 10):
- 5.75 before the treatment
- 1.71 after the last treatment session
- 1.18 two months after completing the therapy
In the placebo group, the pain scores were:
- 5.62 before treatment
- 4.13 after the last treatment session
- 4.12 two months after completing the therapy
Some other studies on this issue haven't been nearly as successful, but as discussed below, it might be related to dose parameters or some other methodological factors in the studies.
5.3. Labial herpes
The researchers of University of Vienna Medical School studied the usage of red light on labial herpes in a double-blind, placebo-controlled trial.[61] There was a 12-fold difference in the median time until the recurrence of herpes symptoms between the groups.(Schindl&Neumann 1999) The subjects were treated in a recurrence-free period. The intervention group were treated for 10 minutes daily for two weeks with visible red light (low-level laser). Placebo group got a similar treatment, but the laser wasn't turned on. The subjects wore masks, so that they couldn't see whether they were given the real treatment. The patients were instructed to return to the department at the time of symptom recurrence. The median recurrence-free interval in the laser-treated group was 37.5 wk compared with 3 wk in the placebo group.
5.4. Wisdom teeth extraction
A 2013 Italian study focused on patients who had their lower third molar removed surgically. The patients were assigned to a LLLT or control group. The active group received near-infrared light to the extraction site and on cheek (980nm).[92] The pain level and edema were measured 24h after the surgery. The subjects in the active group had much less pain and edema compared to the control group. The patients receiving low-level laser therapy reported that their level of pain was 3.75/10 on the next day after surgery. In the control group, the score was 7.1/10.
A couple of other studies on this issue have been published. If we look at the all of the data critically, it isn't completely conclusive, but still very promising.
5.5. Hypothyroidism
This 2013 randomized, placebo-controlled study involving 43 patients with Hashimoto's thyroiditis-induced hypothyroidism was conducted in São Paolo, Brazil.[37] The active group received ten treatment sessions (twice a week) involving the near-infrared irradiation of the skin area close to their thyroid glands (830nm). The placebo group received red light treatment of very low intensity[37] After 10 treatment sessions, the thyroxine (T4) medication was discontinued in both groups. During the next 9 months, the medication was slowly re-introduced if the thyroid hormone levels didn't normalize without medication.
In the control group, hypothyroidism remained after the discontinuation of T4 and the final dosage after the reintroduction of medication was actually higher than before the discontinuation.
However, in the active group, 48% of the patients maintained normal thyroid hormone levels without any medication. The rest also could decrease their dosage a little.
Average T4 dose in the active group (baseline -> 9 months): 93µg -> 39µg
Average T4 dose in the placebo group (baseline -> 9 months): 90µg -> 107µg
In the active group, some other positive changes were also noticed (thyroid volume, TPO antibodies and echogenicity index).
In my personal opinion, this is a very remarkable result. The Brazilian researcher had also conducted a pilot study before this randomized study, with equally positive results. Similar effects have also been reported in some Russian and Ukrainian dissertations (not translated to English).[36-43]
If you are interested in this topic, see also my more recent article Hypothyroidism: Could it be treated with LIGHT?.
6. The systemic anti-inflammatory effect
Usually the red/near-infrared is applied locally to the treatable tissue. If the patient suffers from knee osteoarthritis, then the light is going to be shone on the knee, and so on.
However, light also has systemic effects which seem to be transmitted mainly by circulation of blood. The researcher Natalya Zhevago has conducted an interesting study, in which the patients got some visible light and infrared to the sacral area (low back).[86] The light was quite similar to sunlight, except that it didn't contain UV radiation or blue light, and the infrared portion was polarized. According to one study, polarization of light enchances the metabolic effect slightly.[87] The subjects' blood was analyzed after the treatment. The results were interesting. Subjects' pro-inflammatory cytokines (TNF-α, IL-6 etc.) were dramatically reduced in the subjects, especially in those with initially high values. Also, the concentrations anti-inflammatory cytokines increased.[86] A dramatic decrease in the level of pro-inflammatory cytokines TNF-α, IL-6, and IFN-γ was revealed: at 0.5 h after exposure of volunteers (with the initial parameters exceeding the norm), the cytokine contents fell, on average, 34, 12, and 1.5 times[...]
Another research group reported that polarized light protects rabbits from atherosclerosis when targeted on the outside surface of ear.[88]
"Lovastatin (0.002% in diet) or daily 5-min or 20-min PLT [polarized-light therapy] on the outside surface of ears was started 2 weeks after induction of hypercholesterolemia. [...] [T]he anti-atherosclerotic activity of PLT was superior to lovastatin: 5-min and 20-min PLT decreased the plaque area to 42.2% and 26.4%, respectively." "Importantly, 5-min PLT also exerted a remarkable efficacy in LDL reduction"
The possible mechanisms of these systemic effects hasn't been studied widely yet. One research group conducted an interesting in vitro study and suggested that the effect might be mediated by some growth factors.[89] In the present study, increased growth factors by indirect irradiation stimulate the ERK phosphorylation in the presence of LPS. Especially, indirect 635 nm irradiation can affect MAPK activation and is correlated with the inhibition of pro-inflammatory cytokine expression. The effects observed by Zhevago were quite opposite to the typical effects of UV radiation, which increases TNF-α, IL-6 and other pro-inflammatory cytokines. Sun-exposure is also related to increased IL-6 levels.[90,91]
In human studies, large doses of IL-6 and TNF-α have been demonstrated to suppress peripheral thyroid hormone metabolism by decreasing T3 and increasing rT3.[92,93] We could also speculate, whether lack of sufficient therapeutic light could be one cause of the "rT3 dominance" and hypothyroid symptoms. In two studies, half of the hypothyroid patients getting near-infrared treatment did not require any medication through the 9-month follow-up after the treatment period, somewhat establishing the importance of light for thyroid health.[36,37] Moreover, in a Russian study (Kovalyova 2002), the diabetic patients' total cholesterol was apparently reduced from 7.98 to 5.31 in one month, a change also seen in thyroid treatments.[32-35,94]
7. Light sources (laser, LED, light bulbs, heat lamps, sunlight, therapeutic devices)
"Many people who came to cloudy Eugene to study, and who often lived in cheap basement apartments, would develop chronic health problems within a few months. Women who had been healthy when they arrived would often develop premenstrual syndrome or arthritis or colitis during their first winter in Eugene." - Ray Peat
Laser and LED: Nowadays red light and near-infrared are studied mostly with low-level laser devices (LLLT), but many researchers also use light-emitting diodes (LED) or other light sources. The coherence and pulsing of light (Laser vs. other sources): Although most of the researchers have been using coherent light (laser), it seems that coherence is not a requirement for the beneficial effects. This is logical considering the studies utilizing light-emitting diodes. The idea is also supported by Kellogg's reports, which were based on the usage of incandescent bulbs and arc lights.[2,95] However, it is possible that non-coherent light sources might work best with different parameters than laser (power density, total energy, etc.). According to some data I've seen, laser might reach deep tissues better than other types of light. Laser devices also often emit pulsed light, but it's not clear whether pulsing is very meaningful.[96]
Here are some quotes from an accomplished Estonian LLLT researcher Tiina Karu:
"[T]he stimulative action of various bands of visible light at the level of organisms and cells was known long before the advent of the laser. Also, specially designed experiments at the cellular level have provided evidence that coherent and noncoherent light with the same wavelength, intensity, and irradiation time provide the same biological effect [11-13]. Successful use of LED's in many areas of clinical practice also confirms this conclusion."-Tiina Karu (2003)
"According to action spectra, optimal wavelengths are 820-830, 760, 680, and 620 nm. Large volumes and deeper layers of tissues can successfully irradiated by laser only (e.g. inner and middle ear diseases, injured siatic or optical nerves, deep inflammations etc.). The LED's are excellent for irradiation of surface injuries." -Tiina Karu (year?)
Sunlight: When I was writing my Circadian Rhythms essay, I used to think about the possible explanations of the therapeutic effects of walking outdoors. Sunlight can increase the production of vitamin D and it can also suppress melatonin, but now we have a brand new mechanism that could explain why it's good to spend time outdoors.
There is an interesting correlation between latitude and mean blood cholesterol levels.(Grimes et al. 1996)
A review article on this subject states that in central Europe, the amount of IR-A radiation is limited to 20mW/cm2, which is actually quite a good amount compared to the power output of usual laser devices. However, the sunlight is not monochromatic, which probably increases the dose requirements.[1] Especially in Northern countries, it seems that sunlight is correlated to better health. In some countries, the average cholesterol level of population depends largely on the season. In the Great-Britain, for example, the changing of winter into summer has been shown to decrease cholesterol by 0.8mmol/L (30mg/dl). Also, there seems to be a significant correlation between latitude and cholesterol levels.[97-102]
"In all groups, we found a strong seasonal effect, with 5%–10% higher [total cholesterol] concentrations in winter compared to the summer."[102]
While providing a significant amount of terapeutic wavelengths, sunlight also contains some harmful UV-radiation and blue light, which might decrease the benefits a little bit. Incandescent, halogen and heat lamps: These types of indoor lamps also provide a significant amount of red light and near-infrared. Some of these lamps have internal reflectors, so the light is targeted at one direction. Heat lamps by Philips or Osram have quite a good spectrum with low amount of blue light, but a large amount of their power is emitted as warming IR-B radiation, and only ~12% of the power is emitted as the therapeutic wavelengths (600-1070nm). However, the heat lamps are often high power (up to 250W), so they still emit quite a significant amount of therapeutic wavelengths and might have health effects.
4100K halogen lamp seems to be good source of the therapeutic wavelengths. Sunlight and incandescent/heat lamps also provide significant amounts of 600-1070nm light. (Image source: Heelspurs)
Incandescent lamps and especially halogen lights seem to emit quite a significant amount of their energy as therapeutic wavelengths (up to 35%). They are also quite cheap, which might make them a wise choice for light therapy. In my country, the price of Osram halogen lamps (30-40W) with reflector is about 5€ (~5-6 dollars).
However, there is probably no scientific research testing these kinds of lamps, so it's not proven whether they really work. The preliminary reports by Kellogg were very promising, but as I already mentioned, nowadays almost every research group uses either laser or LED, probably because they don't emit any heat and they are monochromatic or at least the bandwidth is quite narrow.
Energy saving lamps: Because of the phase-out of incandescent lamps, it will soon be increasingly difficult to buy incandescent lamps. It's somewhat sad that they are going to be replaced with compact fluorescent lamps (CFL), which emit some UV but only low amounts of protective red and near-infrared light. This is the reason why some of the researchers, such as Richard Funk and Alexander Wunsch, who also appeared in the Bulb Fiction documentary, have stated that increase in the CFL usage might be harmful to eyes.
Infrared saunas: The possible benefits of infrared saunas aren't usually based on this aforementioned mechanism, because even the shortest-wave infrared saunas don't go below 1400nm. However, the warming effect of far-infrared might also have some beneficial effects.[103]
Infrared lamps:
In theory, a cheap incandescent or halogen lamp with an internal reflector could provide all the pro-metabolic effects, yet nobody has studied the issue (since Kellogg). Some lamps are being sold as "infrared lamps". For example, Beurer models IL30 and IL50 seem to be quite popular models in my country. According to one source, they emit 780-1400nm wavelengths, so they might be suitable. However, no scientific data exists on these.
Therapy devices: The good aspect of lasers and LED lights is that the wavelength can be narrowly chosen (monochromatic light). For example, 830nm has proven good in many studies, and the wavelength is invisible to the eyes. Therefore it'd theoretically make a good and non-disturbing therapy device.
Here is a list of some commercial light devices, some of which have been used in LLLT/light therapy studies: Anodyne, Bioptron, HairMax LaserComb, Omnilux, Noveon NaiLaser, Biolight, Quantum Warp, Syrolight BioBeam, HIRO 3.0, Picasso Lite, HELBO® TheraLite Laser, Super Lizer, BioPhotas Celluma, TinniTool EarLaser and Mustang 2000.
The problem with commercial devices is the fact that they are expensive yet a typical halogen spot lamp (eg. Osram Classic Superstar R50 or R63) could theoretically be as good therapeutically, since the lamps also provide a great amount of the healthy wavelengths.
8. Does it really work? Trying to understand the contradictory results:
Negative results: Even though most of the studies on LLLT have reported positive effects, there have been many studies in which LLLT didn't bring any better results than placebo.
Swedish researchers Jan Tuner and Lars Hode have published an article It's All in the Parameters: A Critical Analysis of Some Well-Known Negative Studies on Low-Level Laser Therapy, in which they explained why, in many cases, the negative results can be explained by the bad parameters used in the study.[104-106]
One typical factor is using a very low dose or power density (eg. 0.004J, 0.005J/cm2), though in some cases too high dose might also block the beneficial effect. Sometimes the problem might arise from targeting the light at the wrong place, using non-effective wavelenghts (eg. 730, 980nm), using the patient as his own control (not taking the systemic effect into account), using an inappropriate control group, or using a malfunctioning device (see below).
One year ago, I tried to discuss some of the LLLT studies with one scientist who works with the doctors who write national recommendations on medical treatments of various diseases. We looked on studies, in which they treated osteoarthritis with LLLT. Most of the results were positive, but in one study condicted in University of Ottawa didn't report a beneficial effect even though the parameters seemed generally "good" on the paper. I couldn't explain this effect to the officer, but later I understood that the light dose they gave to the patients was quite low (0.12J per joint). This is a possible explanation for the negative results of this study.[105,107]
Osmangazi University also conducted a study with negative results.[108] The parameters were similar to the aforementioned Hungarian study with great results.[73] I couldn't find a simple explanation for the negative result, but it could be related to the fact that the diameter of the laser ray was 1mm, and if such a small ray is directed at the wrong point of the joint, then there might be no effect. In one systematic review it was noted that usually the studies using greater ray had better results.[73,108,109]
"Lack of evidence": LLLT isn't still a widely known subject, and therefore many probably think there is no research on the subject. However, a PubMed search with keyword LLLT provides 4000 results, and many meta-analyses have already been published.
Here are some examples of the recent systematic reviews and/or meta-analyses:
Breast cancer-related lymphedema (2015)
Exercise performance and recovery (2015)
Oral mucositis (2014)
Pain relief (2010)
Shoulder tendinopathy (2015)
Temporomandibular disorders (2015)
However, despite the existence of many high-quality RCT trials and good meta-analyses, there is also a really huge amount of problematic research with a high risk of methodological bias. Because of some low-quality studies, some people think that all research on LLLT is bad:
"Like acupuncture, there is a huge literature (4000 on the Pubmeds) of mostly poorly done studies, some showing effect, some not. The Cochrane reviews were not supportive of laser therapy, but note the studies are uniformly lousy."
I think the quality problem is most obvious in the older studies, while nowadays the research has better quality standards. In an 2014 meta-analysis examining the effects LLLT on oral mucositis, most of the studies published after 2011 were of high quality, while older studies had more methodological issues.[72]
"Like acupuncture, better studies demonstrate decreasing effects."
This is also untrue. In a recent meta-analysis on LLLT and shoulder tendinopathy, the average PEDro score of in the studies reporting positive results was 7.5, while the score was 7.6 in the studies reporting negative results.[152]
Also, half of the studies reporting negative results were conducted using a malfunctioning laser device (Roland Pagani) that emitted less than 1% of the output power stated by the manufacturer. Therefore, it can be actually said the studies that reported positive effects were actually better quality.
When I tried to read Wikipedia article on LLLT, I found it difficult to understand the claim that LLLT "may be mildly effective, but in most cases no better than placebo in relieving [...] osteoarthritis, [...] acute and chronic neck pain", since the systematic reviews referenced in Wikipedia article were showing moderate-quality evidence of the beneficial effect.[110-112]
Meta-analyses: When reading meta-analyses on LLLT, some problems might arise. One problem is that so much research is being published nowadays, that there usually exists new data on the subject of the meta-analysis you're reading.
Also, the studies are very heterogenic because the different research groups are using different parameters (wavelength, power, power density, pulsing, dose, treatment time). In one systematic review, in one study patients received 0.3J of light energy per joint, while in another study the dose was 480J per joint (1600-fold difference). Only the latter study reported positive results. However, this doesn't mean that higher energy is better in every case. Some studies have reported a biphasic dose-response curve.[7,113]
In some meta-analyses, there might also be problems related to cherry picking or data synthesis.[114]
9. Light therapy: Animal studies
Red light and near-infrared have also been extensively studied using different animal models. Here is a short list of some research on the subject. The results have been generally very positive:
Rats:
The 2011 review article is mostly focused on how light could affect brains. Most of the research data is from animal studies. (Rojas&Gonzalez-Lima 2011)
Acute joint inflammation [115]
Bone metabolism [116]
Burns [117-120]
Cortical metabolic capacity and memory retention[121]
Diabetic retinopathy [122]
Diabetic kidney [123]
Heart failure-related inflammation [124]
Hypertension [125]
Kidney injury [126]
Laryngitis [127]
Lung injury [153]
Methanol-induced eye injury [128]
Myocardial infarction (infarct size) [129]
Palatal wound healing [130]
Peripheral nerve regeneration [131]
Reflux laryngitis [132]
Rheumatoid arthritis [133]
Skeletal muscle injury [134]
Tendon healing [135]
Traumatic brain injury [136]
Zymosan-induced arthritis [137]
Mice:
Encephalomyelitis [138]
Snake venom poisoning [139]
Traumatic brain injury [140]
Dogs:
Hair regrowth (non-inflammatory alopecia) [141]
Myocardial infarction [142]
Recovery from surgery (hemilaminectomy) [143]
Sperm motility [144]
Rabbits:
Embolic stroke [145]
Wound healing [146]
10. Conclusions
Nowadays, the knowledge of the physiological effects of light is mainly limited to blue light's effects on circadian rhythm, yet the importance of red and near-infrared light is also likely to be a very important factor. Research indicates that red light and near-infrared appear to have quite a wide range of therapeutic effects. Light is very cheap to produce, so there is a possibility that in future, red light would be used as a very cost-effective treatment for various wounds, injuries and chronic diseases.
Link to the article here

 ].
].