Leòmhann
Jedi Master
Just received a govt alert message (signed up for a bunch of govt emergency alerts recently because of some Fed agent activity last week occurring in a nearby building across from us) containing the info below.
Since this is the first that I have heard of this kind of vaccine delivery - and kind of eerily disturbing news at that - I forwarded the email alert to the SoTT email address and am posting here, also. (It may be news in general because searches on SoTT and on the Forum yielded no matching results):
Flu Shots No More?
NIAID Grantees Use New Skin Patches to Deliver Flu Vaccine in Mice
_http://www.niaid.nih.gov/topics/Flu/Research/vaccineResearch/Pages/FluShotsNoMore.aspx
Since this is the first that I have heard of this kind of vaccine delivery - and kind of eerily disturbing news at that - I forwarded the email alert to the SoTT email address and am posting here, also. (It may be news in general because searches on SoTT and on the Forum yielded no matching results):
Flu Shots No More?
NIAID Grantees Use New Skin Patches to Deliver Flu Vaccine in Mice
_http://www.niaid.nih.gov/topics/Flu/Research/vaccineResearch/Pages/FluShotsNoMore.aspx
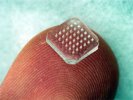
You've probably heard of patches for delivering nicotine-replacement therapy or hormones for birth control. But what about other uses, such as vaccination? For many years, researchers have been working to find a way to deliver flu vaccine—whose components are much larger than those of nicotine and hormones—using a transdermal (across the skin) patch. One method, developed by scientists at the Georgia Institute of Technology and Emory University, uses a patch made of tiny, painless microneedles that dissolve and allow flu vaccine to pass through the skin.
A 2010 study by Mark Prausnitz, Ph.D., and Sean Sullivan, Ph.D., of Georgia Tech, and Dimitrios Koutsonanos, M.D., Ioanna Skountzou, M.D., Ph.D., and Richard Compans, Ph.D., of Emory University, compared microneedle patches to traditional hypodermic needles to vaccinate mice against the flu. The microneedles are made of a nontoxic polymer called polyvinylpyrrolidone and were developed by the group at Georgia Tech. The studies detailing the immune system reactions to microneedle vaccine were led by the scientists at Emory University. The research was supported by NIAID grants, by NIAID's Centers of Excellence for Influenza Research and Surveillence program, and by the National Institute of Biomedical Imaging and Bioengineering (NIBIB).
Microneedle Patches: A Melding of Engineering and Biology
The investigators at Georgia Tech used an innovative method to create the microneedle patches. In a process known as in situ polymerization, they mixed liquid vinylpyrrolidone with the vaccine, poured the mixture into a microneedle mold, and exposed it to ultraviolet light. This induced polymerization, creating much larger molecules.
Once placed on the skin, the microneedles pass through the surface skin layers, moisten, and dissolve, delivering flu vaccine to antigen-presenting cells in the skin. These cells then break down antigens and display them to other immune cells. The body mounts an immune response to those antigens, and is thereby prepared to fight off the virus in the future. When the microneedles fully dissolve—within a few minutes—the patch can be removed and discarded.
Testing Immunity from Microneedle-Delivered Vaccine
After vaccination, researchers at Emory University measured flu antibody levels in the blood and found no difference between mice that received the microneedle vaccine and those that received a hypodermic injection. In fact, when microneedle-vaccinated mice were exposed to flu virus, they were significantly better protected than those that received a hypodermic injection. Four days after exposure, mice in the microneedle group were able to clear the virus out of their lungs 1000 times more efficiently than mice in the hypodermic group.
"Viral load is an important measure because it addresses the source of the problem: virus in the lungs. Microneedle vaccination brought the viral load in the lungs almost to zero," says Dr. Prausnitz. A reduced viral load may also have implications for the infectivity of flu; if a person expels less virus with a cough or sneeze, transmission may be reduced.
Microneedle patches also have practical advantages over traditional hypodermic injections: they take up less space in clinics, do not require special disposal (as hypodermics do), are inexpensive to make, and may be simple enough for patients to self-administer at home. If results from animal studies can be replicated in humans, not only would people who receive the vaccine be better protected from the flu, but it may be easier for more people to get vaccinated. If more people are vaccinated, fewer people are likely to get sick and be able to pass the virus on to others, lowering everyone's chances of being exposed.
Looking to the Future
The researchers continue to explore why microneedle delivery appeared to produce better results than standard injections. Microneedle skin patches target a different set of immune cells than conventional intramuscular injection, notes Dr. Prausnitz. "I don't think the improvement in immunogenicity is something unique to microneedles, but rather is unique to delivery through the skin. Microneedles enable that to take place," he says.
In 2012, two publications from the Emory and Georgia Tech teams continued to advance the field. Research published in the journal mBio showed that their hunch about the importance of transdermal delivery was correct. They found evidence that microneedles prompted an immediate flood of immune proteins called cytokines to the skin near the site of the patch. Cytokines help recruit a variety of immune cells involved in generating a defensive response to the inactivated virus in the vaccine.
In a study published in Scientific Reports, the scientists tested whether the protection conferred by microneedle vaccination would be long-lasting. They administered a single dose of a licensed flu vaccine via a microneedle patch to mice and compared their antibody responses with those of mice receiving injections in muscle. The microneedle delivery prompted a longer lasting and more robust antibody response, the investigators found. The microneedle also prompted a response from the immune system's cellular responders (distinct from the antibody response) although the cellular response was similar to that seen in mice receiving flu vaccine in muscle.
Finally, the teams looked at how long protection from flu infection lasted in each group of vaccinated mice. When exposed to high levels of mouse-adapted flu virus 4 weeks after vaccination, all mice were fully protected against fatal infections. But when the researchers waited 36 weeks before exposing mice to virus, only the microneedle-vaccinated animals were fully protected. All of the microneedle-vaccinated mice survived virus challenge, while 40 percent of the conventionally vaccinated mice died.
The investigators hope to begin early stage clinical trials of the flu microneedle patch in people by 2015.
References:
SP Sullivan et al. Dissolving polymer microneedle patches for influenza vaccination. Nature Medicine DOI: 10.1038/nm.2182 (2010).
M Del Pilar Matin et al. Local response to microneedle-based influenza immunization in the skinExternal Web Site Policy. mBio DOI:10.1128/mBio.00012-12 (2012).
D Koutsonanos et al. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Scientific Reports DOI: 10.1038/srep00357 (2012).