Given that Laura and others have been talking about probiotic enemas recently (start reading here), the following articles might be helpful in connection to that, IF you have eye problems. They suggest that one should not only look for causes related to the common ocular diseases. The root cause might be, as often happens, in your gut!
Ocular manifestations of inflammatory bowel disease.
(The whole text is not available for free).
Ocular complications of inflammatory bowel disease.
Full text here.
Managing IBD outside the gut: ocular manifestations.
(The whole text is not available for free).
The prevalence of ocular involvement in patients with inflammatory bowel disease.
In uveitis, bacteria in gut may instruct immune cells to attack the eye
From the gut to the eye: commensal microbes as potential triggers of autoimmune uveitis
Ocular manifestations of inflammatory bowel disease.
Abstract
The association between inflammation of the eyes and the intestine is not often recognized by ophthalmologists. We report two patients who developed peripheral corneal ulcers, episcleritis, and scleritis just prior to the onset of Crohn's disease. The severity of the eye disease paralleled that of the intestinal symptoms, and both conditions subsided after treatment with topical steroids, oral prednisone, oral sulfasalazine, and hydrocortisone retention enemas. Inflammatory bowel disease should always be included in the differential diagnosis of scleritis and uveitis, as the patient may be benefited greatly by appropriate, early therapy of this gastrointestinal disorder.
(The whole text is not available for free).
Ocular complications of inflammatory bowel disease.
Abstract
Though inflammatory bowel disease (IBD) has a specific predilection for the intestinal tract, it is a systemic inflammatory disorder affecting multiple organs, including the eye. Ocular complications directly related to IBD are categorized as primary and secondary. Primary complications are usually temporally associated with IBD exacerbations and tend to resolve with systemic treatment of the intestinal inflammation. These include keratopathy, episcleritis, and scleritis. Secondary complications arise from primary complications. Examples include cataract formation due to treatment with corticosteroids, scleromalacia due to scleritis, and dry eye due to hypovitaminosis A following gut resection. Some ocular manifestations of IBD can lead to significant visual morbidity and temporally associated complications can also be a herald of disease control. Furthermore, ocular manifestations of IBD can occasionally manifest before the usual intestinal manifestations, leading to an earlier diagnosis. Thus, it is important to understand the clinical presentation of possible ocular manifestations in order to initiate appropriate treatment and to help prevent significant visual morbidity.
Full text here.
Managing IBD outside the gut: ocular manifestations.
Abstract
Extraintestinal manifestations are common in inflammatory bowel disease (IBD), being reported in over 25% of patients. Ocular complications of IBD occur in around 10% of cases, but may precede systemic symptoms and are usually nonspecific. Complications of therapy, such as cataracts or glaucoma from steroid use or keratoconjunctivitis sicca related to 5-aminosalicylic acid medications, may also involve the eyes. The pathogenesis remains unclear, but factors such as the extent of intestinal disease, disease activity, and the presence of associated arthritis have been associated with ocular involvement. Conjunctivitis, episcleritis, scleritis and uveitis are by far the most common ophthalmic complications of IBD. However, posterior uveitis, intraretinal hemorrhages, vasculitis, choroiditis, optic neuropathy, and vaso-occlusive phenomena may also occur. The most frequent severe ocular manifestation is anterior uveitis (more common in women). It usually presents as a mild anterior nongranulomatous uveitis (60% of the cases). The inflammation in the eye and the inflammation in the gut are rarely correlated. Patients with uveitis, scleritis, and other anterior segment inflammation usually respond to steroids (topical, periocular or systemic). If the inflammation is resistant to steroids, or if appreciable steroid adverse effects are encountered, systemic immunosuppressive treatment should be considered; this is more likely in HLA-B27-positive patients with uveitis. Evaluation of the eye should be a routine component in the care of patients with IBD.
(The whole text is not available for free).
The prevalence of ocular involvement in patients with inflammatory bowel disease.
Abstract
PURPOSE:
The aim of this prospective randomized clinical study was to evaluate the prevalence of ocular involvement in patients with inflammatory bowel disease (IBD).
MATERIALS AND METHODS:
We prospectively evaluated 116 patients who went to the gastroenterology clinic with endoscopically proven IBD between December 2001 and February 2005. All patients were examined for evidence of ocular manifestations of IBD. Twenty patients had Crohn's disease and 96 had ulcerative colitis. The examination consisted of slit-lamp examinations, tonometry, visual acuity, and indirect ophthalmoscopy.
RESULTS:
The mean age of the 116 patients with IBD who were enrolled was 40.6 +/- 14.4 years (range 16 to 75). Twelve of 20 patients (60%) with Crohn's disease and 22 of 96 patients (22.92%) with ulcerative colitis had ocular involvement. The most common ocular findings were conjunctivitis (8.62%) and blepharitis (6.9%) followed by uveitis (5.17%), cataract (5.17%), and episcleritis (3.45%). Extraintestinal complications were seen in 12 (35.3%) of 34 patients with ocular involvement and in 16 (19.5%) of 82 patients without ocular involvement.
CONCLUSION:
Because the ocular complaints of IBD patients are often nonspecific, it may be helpful to performed eye examinations as a routine component in the follow-up of these patients. It is well-known that early diagnosis and treatment of ocular involvement may prevent serious ocular complications that could be associated with significant visual morbidity. In addition, clinicians should be aware that some ocular diseases, such as uveitis and scleritis, might precede a diagnosis of ulcerative colitis or Crohn's disease.
In uveitis, bacteria in gut may instruct immune cells to attack the eye
The inflammatory eye disorder autoimmune uveitis occurs when a person’s immune system goes awry, attacking proteins in the eye. What spurs this response is a mystery, but now a study on mice suggests that bacteria in the gut may provide a kind of training ground for immune cells to attack the eye. The study was conducted by researchers at the National Eye Institute (NEI), part of the National Institutes of Health.
Evidence increasingly suggests that there is an association between the microbiota in the gut – bacteria, fungi and viruses – and the development of autoimmune disorders. Findings from this study suggest how that association may be made and therefore have implications about the origins of autoimmune diseases not only in the eye, but also elsewhere in the body, said Rachel R. Caspi, Ph.D., a senior investigator at NEI whose lab led the study.
Autoimmune uveitis accounts for more than 10 percent of severe visual disability in the United States. Corticosteroids provide a blanket approach to the disorder by quelling inflammation, but their long-term use can lead to adverse side effects.
Understanding what spurs autoimmune uveitis is fundamental to the development of safer long-term therapies and possibly even strategies for preventing it, said Reiko Horai, Ph.D., a staff scientist at NEI and a lead author of the study, published in the journal Immunity. Carlos R. Zarate-Blades, Ph.D., a postdoctoral fellow at NEI, is the other lead author.
The eye is one of the places in the body that has immune privilege meaning it is protected by a blood-tissue barrier that physically separates it from the rest of the body and minimizes the exchange of substances and blood-borne cells going in and out of the eye.
In the case of autoimmune uveitis, immune cells (T cells) are thought to penetrate through this blood-ocular barrier. But first, they must become activated, which occurs when they come in contact with the protein that they are pre-programmed to recognize. This is how T cells fight an infection and some types of cancer – by targeting proteins on bacteria, viruses and cells. And herein lies a paradox that’s been puzzling uveitis researchers. The proteins believed to be targeted in autoimmune uveitis are sequestered in the eye; they don’t exist elsewhere in the body. So what activates the T cells and allows them to cross the blood-ocular barrier?
The researchers asked this question by studying mice genetically engineered to develop autoimmune uveitis, due to a high level of retina-reactive T cells in their bodies. Before the mice had developed signs of the disease, the team searched their bodies for activated T cells and made an interesting discovery. Levels of activated T cells were not elevated in the lymph nodes (the glands that tend to swell during infections), but they were abundant in the intestines. What’s more, the T cells in the gut produced a protein shown in previous studies by Dr. Caspi’s team to augment the damage in autoimmune uveitis.
“These discoveries support the idea that activation of T cells in the gut may actually precede the first signs of the disease,” she said. To test that idea, the researchers gave the mice an antibiotic cocktail designed to wipe out a broad spectrum of bacteria in the gut and by rearing them in a germ-free environment. They found that mice without gut bacteria developed autoimmune uveitis much later, and with less severity, compared to control mice with normal gut flora.
There was a similar delay in uveitis and decline in its severity when the uveitis-prone mice were raised in an environment free of bacteria and other germs. But when the same mice were later moved into normal housing, where they acquired normal gut bacteria, the uveitis roared in at full strength.
So how do bacteria in the gut activate T cells against cells in the eye? The researchers theorize that bacteria in the gut produce a molecule that, to T cells, looks similar to a protein in the retina. This gives the T cells marching orders to look for that retinal protein and attack it. Consistent with this idea, the researchers found that they could activate retina-specific T cells by exposing them to a soup of bacterial proteins extracted from mouse intestines. When those activated T cells were injected into normal mice (not prone to uveitis), the mice developed uveitis.
“Given the huge variety of bacteria in our intestines, if they can mimic a retinal protein, it is conceivable that they could also mimic other self-proteins in the body. So we believe that normally harmless bacteria in the gut could be involved in promoting other autoimmune diseases as well,” Dr. Caspi said.
The results don’t have immediate implications for patients, but will help inform further research to understand the disease and help to develop new therapies, Dr. Caspi said. Eliminating bacteria from our bodies isn’t a treatment option, and a bacteria-free state would not be feasible. However, if scientists could one day identify the bacteria specifically involved in promoting autoimmune uveitis, it might be possible to target only those of interest.
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov.
From the gut to the eye: commensal microbes as potential triggers of autoimmune uveitis
Autoimmune uveitis is a group of inflammatory diseases that affect the retina of the eye and related tissues and constitute a major cause of human blindness. It is believed that the disease is triggered by the activation of circulating lymphocytes capable of recognizing the proteins (antigens) that are unique to the eye, whereupon they gain the ability to invade the healthy eye and induce uveitis. How and where these lymphocytes become activated has been a long-standing mystery, because the proteins that they are programmed to recognize which would activate them are inside the eye and are not accessible. Questions such as these cannot be studied in humans for reasons that are both ethical and practical (the patient comes to the clinic after disease has already developed). Therefore, researchers rely on animal models to study disease related processes in a setting that can be controlled and manipulated experimentally.
lymphocyte activation
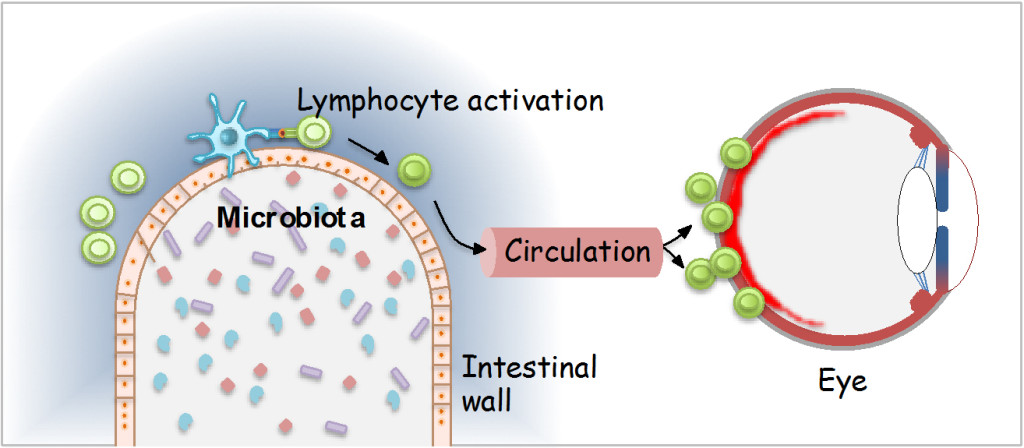
Fig. 1. Schematic representation of lymphocyte activation in the intestine on commensal microbiota, which endows them with the ability to induce uveitis. (Adapted from press release by the National Eye Institute).
In traditional animal models of uveitis, experimental mice or rats are immunized with proteins from the eye (incorporated into an emulsion of oil containing heat-killed tuberculosis bacteria, known as complete Freund’s adjuvant, which amplifies immune responses). Their lymphocytes then become activated and the animals develop uveitis. However, these models are not appropriate to study natural causes of disease, as the trigger is provided by the researcher in the form of immunization. We, therefore, developed a new model of autoimmune uveitis in genetically engineered mice that develop the disease spontaneously as a consequence of having an increased number of circulating retina-specific lymphocytes.
By studying these mice, which additionally contained a fluorescent reporter molecule to show lymphocyte activation, we observed that the retina-specific lymphocytes became activated in the intestine. This occurred even before the animals developed clinically apparent uveitis, and was still present in mice that had the retinal antigen genetically deleted. Treatment of the mice with antibiotics, or rearing them in germ-free conditions, resulted in strongly delayed and attenuated development of disease, pointing to bacteria in the intestine as a potential trigger. Indeed, further experiments demonstrated that circulating retina-specific lymphocytes isolated from the genetically engineered mice could be activated by culture with extracts of bacteria-rich intestinal contents. Moreover, these lymphocytes induced uveitis when subsequently injected into genetically normal recipients, whereas lymphocytes that were cultured by themselves, did not. Extracts from germ free animals did not have an activating effect. In the aggregate, these findings indicated that bacteria in the intestine make something (which we identified as a protein) that to the retina-specific lymphocytes “looks” similar to the retinal antigen that they are programmed to recognize. These lymphocytes then enter the circulation and make their way to the eye, which they are able to infilatrate due to their ativated state, and cause uveitis (Figure 1).
Due to the complexity of the intestinal microbiome, it has not yet been possible to identify the responsible organism(s) and to characterize their product. Bioinformatic and biological methods may help us achieve this, but there is still much work to be done. Irrespective of that, however, our findings have clear implications for the etiology of human uveitis. Furthermore, in view of the almost infinite variety of microbes living in and on our bodies, if they can mimic a retinal antigen, it is possible that they may produce substances that mimic tissue antigens involved in other autoimmune diseases. If that is true, commensal microbes might be a more common trigger of autoimmune diseases than is currently appreciated.
While the knowledge gleaned from these studies can help us understand the biology of the disease, the conclusions cannot yet be applied clinically. In view of what we know about the importance of microbiota for proper development and functioning of immunity, host defense and metabolism, prophylactic treatment with broad spectrum antibiotics is not feasible. Furthermore, we are not yet able to identify the individuals at risk for uveitis with sufficient certainty. However, as our knowledge advances and as our ability to control undesirable immunological responses in an increasingly selective fashion develops, specific immunological interventions or introduction of appropriate probiotics and prebiotics might become possible.