Where is c60 found?
An unusual carbon-rich rock believed to be more than 600 million years old has yielded the first evidence that fullerenes occur in nature. The fullerenes
C60 and C70 were
discovered in a sample of shungite,
a rock of uncertain origin found near the Russian town of Shunga, about 250 miles northeast of St. Petersburg.
What are the properties of buckminsterfullerene?
Three forms, or 'allotropes', of pure carbon are
diamond, graphite and buckminsterfullerene (or 'buckyballs'). In all three allotropes, the carbon atoms are joined by strong covalent bonds but in such different arrangements that the properties of the allotropes are very different.
What are buckyballs used for?
Combining buckyballs, nanotubes, and polymers to produce inexpensive solar cells that can be formed by simply painting a surface. Buckyballs may be used to store hydrogen, possibly as a fuel tank for fuel cell powered cars. Buckyballs may be able to reduce the growth of bacteria in pipes and membranes in
water systems.
How are buckyballs used in nanotechnology?
Much of the current research and commercialisation of
nanotechnology relies on tubes, wires and balls made from carbon atoms. ... The carbon based tubes can be carbon nanotubes, buckytubes and very long tubes are often referred to as nanowires. The balls are known as fullerines or
buckyballs.
What do fullerenes do?
A
fullerene is an allotrope of carbon in the form of a hollow sphere, ellipsoid, tube, and many other shapes. Spherical
fullerenes, also referred to as Buckminsterfullerenes or buckyballs, resemble the balls used in association football. Cylindrical
fullerenes are also called carbon nanotubes (buckytubes).
What is the formula of buckyball?
C60
Why is it called buckminsterfullerene?
Buckminsterfullerene is a polyhedral CARBON structure composed of around 60-80 carbon atoms in pentagon and hexagon configuration. They are
named after Buckminster Fuller because of structural resemblance to geodesic domes. Fullerenes can be made in high temperature such as arc discharge in an inert atmosphere.
Who discovered c60?
In 1985 the discovery was announced of a third allotrope in which the atoms form C60 molecules in the shape of a football. This led to the award of the 1996 Nobel Prize to Harry (now Sir Harry)
Kroto of Sussex University,
Robert Curl and
Richard Smalley (both of Rice University in Houston, USA).
Who was the buckminsterfullerene named after?
Naming. The discoverers of the Buckminsterfullerene (C60) allotrope of carbon named it after
Richard Buckminster Fuller, a noted architectural modeler who popularized the geodesic dome. Since buckminsterfullerenes have a similar shape to those of
such domes, they thought the name appropriate.
Is c60 toxic?
In contrast to chemically--either covalently or noncovalently--modified fullerenes, some
C60 derivatives can be highly
toxic. ... This chapter offers a general review of the studies on the
toxicity of [60]fullerene or
C60, the most abundant fullerene, and its derivatives.
Toxicity studies of fullerenes and derivatives. - NCBI
Toxicity studies of fullerenes and derivatives. - PubMed - NCBI
Is fullerene found in nature?
Fullerenes have since been
found to occur in
nature. ... The discovery of
fullerenes greatly expanded the number of known carbon allotropes, which had previously been limited to graphite, graphene, diamond, and amorphous carbon such as soot and charcoal.
What is c60 purple power?
C60 (aka Buckminsterfullerene) is a naturally occurring molecule found in Space and on Earth. ...
C60 Purple Power takes high purity
C60 and cool infuses it into healthy vegetable oils for maximum monomolecular absorption (available infused into Avocado and Coconut oils).
[and available on Amazon]
Is c60 aromatic?
Is C60 buckminsterfullerene
aromatic? ... C(60) does not have "superaromatic" or even
aromatic character, but is a spherically π antiaromatic and enormously strained species. This explains its very large and positive heat of formation (610 ± 30 kcal mol(-1)).
What is the diameter of a buckyball?
The van der Waals diameter of a C60 molecule is
about 1.1 nanometers (nm). The nucleus to nucleus diameter of a C60 molecule is about 0.71 nm.
How is fullerene used in medicine?
Fullerene is able to fit inside the hydrophobic cavity of HIV proteases, inhibiting the access of substrates to the catalytic site of enzyme. It can be
used as radical scavenger and antioxidant. ... In addition,
fullerenes have been
used as a carrier for gene and drug delivery systems.
What is the molecular formula of Buck ministers fullerene?
Chemical Names: Fullerene; Fullerene
C60; 99685-
96-8; Buckminsterfullerene;
C60Fullerene; Buckyball More... Buckminsterfullerene is a polyhedral CARBON structure composed of around 60-80 carbon atoms in pentagon and hexagon configuration.
Does buckminsterfullerene have a high melting point?
Its molecules are made up of 60 carbon atoms joined together by strong covalent bonds. Molecules of C 60 are spherical. There are weak intermolecular forces between molecules of
buckminsterfullerene. These
need little energy to overcome, so
buckminsterfullerene is slippery and
has a low
melting point.
What was the first fullerene discovered?
The first
fullerene molecule to be discovered, and the family's namesake,
buckminsterfullerene (C60),
was manufactured in 1985 by Richard Smalley, Robert Curl, James Heath, Sean O'Brien, and Harold Kroto at Rice University. The name was an homage to Buckminster Fuller, whose geodesic domes it resembles.
Does buckminsterfullerene conduct electricity?
It is very strong because of its large regular arrangement of carbon atoms joined by covalent bonds . Like graphite, graphene
conducts electricity well because it has delocalised electrons that are free to move across its surface.
What allotrope means?
Allotropy or allotropism (from Ancient Greek ἄλλος (allos),
meaning 'other', and τρόπος (tropos),
meaning 'manner, form') is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as
allotropes of these elements.
What are the properties of fullerenes?
Properties of Fullerene Molecules. The three-dimensional spherical fullerene molecule has unique chemical,
physical, and physico-chemical properties, which include the following: The molecule can act as a semiconductor, conductor and superconductor under specific conditions.
What is a c60 molecule?
C60 is a
molecule that consists of 60 carbon atoms, arranged as 12 pentagons and 20 hexagons. ... Depending on the number of hexagons,
molecules of different sizes are obtained. They are called Fullerenes, after the American architect Richard Buckminster Fuller.
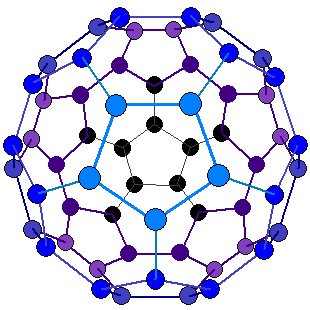
How many pentagons and hexagons are in a buckyball?
Buckminsterfullerene is a type of fullerene with the formula C60. It has a cage-like fused-ring structure (truncated icosahedron) that resembles a soccer ball (football), made of
twenty hexagons and
twelve pentagons, with a carbon atom at each vertex of each polygon and a bond along each polygon edge.
How is carbon 60 made?
C
60 and other fullerenes are now routinely
made by a low pressure method in which an electric discharge is passed across the gap between 2
carbon electrodes in a helium atmosphere. The resulting soot is collected and mixed with a solvent such as benzene; the fullerenes dissolve and can be extracted.
Is buckminsterfullerene a giant covalent structure?
Buckminsterfullerene is yet another allotrope of carbon. It is actually not a
giant covalent structure, but a
giant molecule in which the carbon atoms form pentagons and hexagons - in a similar way to a leather football. It is used in lubricants.
What is buckminsterfullerene used for?
Buckminsterfullerene is a black solid although it is coloured in certain solutions eg deep red when in petrol. The tube fullerenes are called nanotubes which are very strong and are conductors of electricity. Their unusual electrical properties mean that nanotubes are
used as semiconductors in electronic circuits.
Who discovered fullerene an allotrope of carbon?
The scientists who
vaporized the graphite to produce C60 named the new carbon allotrope buckminsterfullerene (shortened to fullerenes or buckyballs) because the geodesic domes designed by inventor and architect
Buckminster Fuller provided a clue to the molecule's structure.
How do carbon nanotubes filter water?
Scientists have created tiny
carbon nanotubes that can
filter water very efficiently, and
could one day help turn seawater into drinkable
water. ... Aquaporins are found all over the human body: they're used to transport
water through membranes and
filter out ions so that cells can remain healthy.
How many carbon atoms are in fullerene?
Buckminsterfullerene was the first fullerene to be discovered. Its molecules are made up of
60 carbon atoms joined together by strong covalent bonds. Molecules of C 60 are spherical.
Why does buckminsterfullerene have a lower melting point than diamond?
They are made up of large molecules but
do not
have a giant covalent structure. Weak intermolecular forces exist between individual buckyballs. Little energy is needed to overcome these forces, so substances consisting of buckyballs are slippery and
have lower melting points than graphite or
diamond .
~~~~~~~~~~~~~~~~
Buckminsterfullerene is a type of fullerene with the formula C60. It has a cage-like fused-ring structure (truncated icosahedron) that resembles a soccer ball (football), made of twenty hexagons and twelve pentagons, with a carbon atom at each vertex of each polygon and a bond along each polygon edge.
Chemical formula: C60
Crystal structure: Face-centered cubic,
cF1924
Appearance: Dark needle-like crystals
Buckminsterfullerene - Wikipedia
Buckminsterfullerene - Wikipedia
The first fullerene molecule to be discovered, and the family's namesake,
buckminsterfullerene (C60), was manufactured in 1985 by
Richard Smalley,
Robert Curl,
James Heath,
Sean O'Brien, and
Harold Kroto at
Rice University. The name was an homage to
Buckminster Fuller, whose
geodesic domes it resembles.
The structure was also identified some five years earlier by Sumio Iijima, from an electron microscope image, where it formed the core of a "bucky onion".[2] Fullerenes have since been found to occur in nature.
[3]More recently,
fullerenes have been detected in outer space.[4] According to astronomer Letizia Stanghellini, "It’s possible that buckyballs from outer space
provided seeds for life on Earth."
[5]

en.wikipedia.org
 ).
).