Ohio pharmacy board reverses ban on hydroxychloroquine as coronavirus treatment after DeWine’s request
Jul 29, 2020
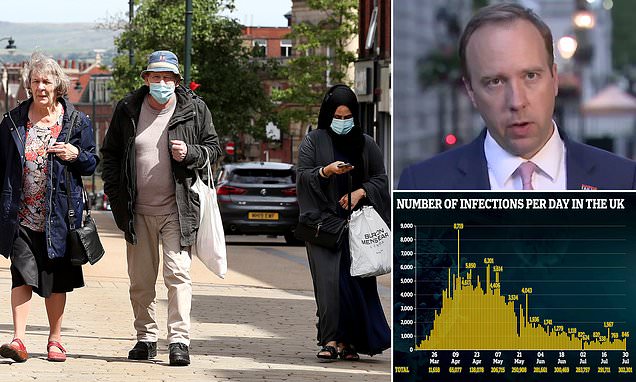
Hydroxychloroquine has been touted by President Donald Trump as a way to treat and prevent the coronavirus. The Ohio pharmacy board planned to ban the drug as a COVID-19 treatment until Gov. Mike DeWine spoke up about it.
The State of Ohio Board of Pharmacy has changed course on its ban of hydroxychloroquine and chloroquine as coronavirus treatments following the governor’s urging to do so.
Beginning Thursday, pharmacies, clinics and other medical institutions were to be prohibited from dispensing or selling the drugs to treat COVID-19, according to
regulations issued by the State of Ohio Board of Pharmacy. They could still be used in clinical trials, said Cameron McNamee, director of policy and communications for the board.
That regulation change has since been pulled back by the board though. Instead, the board now plans to re-examine the issue with the assistance of the State Medical Board of Ohio, clinical experts, and other stakeholders to determine its next steps,
according to an announcement
The board’s shift came after Gov. Mike DeWine asked the state pharmacy board on Thursday morning to rescind its plan to ban hydroxychloroquine and chloroquine as treatments for the virus.
DeWine said the decision of how to treat COVID-19 should instead be between patients and their doctors.
“The Board of Pharmacy and the State Medical Board of Ohio should revisit the issue,
listen to the best medical science, and
open the process up for comment and testimony from experts,” DeWine said in a prepared statement.
[How about applying that to the mask mandate]
Hydroxychloroquine has been touted by President Donald Trump
despite medical studies showing the drug to be ineffective at treating the disease. The drug may also cause serious cardiac side effects, according to the Food and Drug Administration.
[Still pushing the lies]
The U.S. Food and Drug Administration in mid-June revoked an emergency authorization for hydroxychloroquine that had allowed it to be used to treat COVID-19 patients. Despite the FDA’s revocation and until now, it could technically still be used for off-label treatment of the virus in Ohio, McNamee said.
“Basically, it’s a patient safety issue,” McNamee said on Wednesday before DeWine’s request and the board’s subsequent reversal. “We’re looking at the best science to determine what’s best for the patients of Ohio.”
Under the regulation,
pharmacists in Ohio found to be selling or dispensing the drug to treat COVID-19 could have faced disciplinary action ranging anywhere from a warning or fine to a temporary suspension of their license. That action would have depended on the situation, McNamee said Wednesday.
“The long and short of it is, we want people to
focus on what works, such as social distancing and mask use,” McNamee said. “We ultimately want to make sure people are being safe and not exposing themselves to drugs that have shown not to be effective in treating COVID-19.”
[Still in science denial]
The board’s original move to ban the use of hydroxychloroquine to treat COVID-19 came as misinformation continued to spread about it. As recently as this week, Trump has promoted the drug and said he took it as a preventative measure.
McNamee said the board’s initial decision had nothing to do with Trump’s continued endorsement of hydroxychloroquine.
This isn’t the first time the state pharmacy board has stepped in to regulate the use of the drug during the pandemic.
In March, the Ohio Board of Pharmacy cracked down on doctors who were hoarding hydroxychloroquine for themselves, family and friends in case it was needed. At that time, the board implemented restrictions that said the drug could be prescribed only for those who had tested positive for COVID-19.
Full story: