I was revisiting the sites that deal with potassium iodide used for dermatoligic conditions and thought it might be useful to recap some of that material to see if there are some clues.
There is a table that lists conditions and dosage: http://dermnetnz.org/treatments/potassium-iodide.html
In some cases, it's 6 grams a day (!) for up to ten weeks!!!!
SOME people get side effects:
They say there can be issues with "prolonged use" at higher doses. Don't know what "prolonged" means since they've just said that some conditions need up to 10 weeks of treatment at very high doses!!!
With prolonged use, patients may experience symptoms of iodism or potassium toxicity.
Following a link there is this:
http://dermnetnz.org/reactions/halogenodermas.html
See also: http://emedicine.medscape.com/article/1090031-overview
This: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3754371/
This: http://www.ncbi.nlm.nih.gov/pubmed/7458376
Here's a page about EN: http://www.medicinenet.com/erythema_nodosum/article.htm
And people telling their stories: http://www.medicinenet.com/erythema_nodosum/patient-comments-287-page2.htm
It's pretty horrible and it seems like what was said in one of the articles cited above might be the cause:
"Halogens may make usually non-pathogenic or harmless skin microorganisms pathogenic."
And not just in the skin, but throughout the body. When the iodine starts kicking that stuff out, apparently one can get quite sick in a number of ways. My guess is that it is not the iodine or potassium iodide making the person sick, but the displaced/detoxing of the bromides/fluorides. But that can be very problematical!
Perhaps the peeps who get some of these reactions should switch over to potassium iodide and back off the lugol's? They mention several times the "immunomodulatory features" of potassium iodide. Maybe it's the combination of potassium and iodine in the potassium iodide formulation that is key?
Potassium iodide
Potassium iodide (KI) is prepared by reacting iodine with a hot solution of potassium hydroxide. It is mainly used in the form of a saturated solution, 100gm of potassium iodide to 100ml of water. This equates to approximately 50mg/drop. The solution is usually added to water, fruit juice or milk before drinking.
Potassium iodide has been primarily used in the treatment and prevention of simple goitre, which is endemic in areas where the diet is deficient in iodides. Goitre results from low levels of thyroid hormone. Potassium iodide is usually given for this purpose as iodised salt. Other indications include treatment of hyperthyroidism, radiation protectant of the thyroid gland, pre-operative preparation of patients with Graves disease and the treatment of some dermatological conditions such as cutaneous lymphatic sporotrichosis and inflammatory dermatoses.
Potassium iodide for dermatological diseases
It is not clear how potassium idodide works in the treatment of dermatological conditions but it may be because of its effect on neutrophils. Neutrophils are a type of white blood cell, important in the immune system's fight against bacteria.
Potassium iodide appears to be particularly effective in conditions where neutrophils predominate in the early stages of the disease. Its activity against fungi is possibly because it kills the fungi directly or by enhancement of the body's immunologic and non-immunologic defence mechanisms.
The effectiveness of potassium iodide in the treatment of dermatological diseases has been shown in a number of studies.
There is a table that lists conditions and dosage: http://dermnetnz.org/treatments/potassium-iodide.html
In some cases, it's 6 grams a day (!) for up to ten weeks!!!!
SOME people get side effects:
Side effects are rare when potassium iodide is used in short courses and at low doses. The most common side effects are abdominal pain, diarrhoea, nausea or vomiting. These acute side effects often go away during treatment as your body adjusts to the medicine or can be lessened by avoiding rapid dosage increases. Other, less common side effects include urticaria and angioedema, i.e. hives, swelling of arms, face, legs, lips, tongue, throat and lymph glands.
They say there can be issues with "prolonged use" at higher doses. Don't know what "prolonged" means since they've just said that some conditions need up to 10 weeks of treatment at very high doses!!!
With prolonged use, patients may experience symptoms of iodism or potassium toxicity.
Symptoms of iodism include:
Burning of mouth or throat
Metallic taste
Increased watering of mouth
Sore teeth and gums
Severe headache
Symptoms of potassium toxicity include:
Confusion
Irregular heartbeat
Numbness, tingling, pain or weakness in hands or feet
Following a link there is this:
http://dermnetnz.org/reactions/halogenodermas.html
Halogenodermas
What are halogenodermas?
Halogenodermas are rare skin reactions related to high levels of halogens in the body. Halogens are a group of natural elements with similar chemical properties. Examples of clinically relevant halogens are bromine and iodine, and when these are part of compounds, such as medications, they are called bromides and iodides. The skin eruption may be named for the specific halogen involved, i.e., bromoderma and iododerma respectively.
Who gets a halogenoderma?
Bromine and bromides
Bromides have been widely used orally as sedatives, anti-epileptics, anti-neoplastics (chemotherapy), spasmolytics (used for colic) and expectorants (cough medicine).
Bromide may still be prescribed in:
carbromalhydroxyzine hydrochloride (short acting sedative)
pipobroman (alkylating agent for myeloproliferative disorders, e.g., some types of leukaemia)
potassium bromide (for epilepsy)
ipratropium bromide (bronchodilator)
dextromethorphan hydrobromide (antitussive in cough mixtures)
scopolamine bromide (to treat colic in infants).
Bromoderma has been reported in breastfed infants when the mother was taking a bromine-containing medication.
Bromine intoxication may occur in 1-10% of exposed patients.
Citrus-flavoured soft drinks may contain brominated vegetable oil as an emulsifier and flavour carrier. Excessive consumption of cola drinks and ‘Ruby Red Squirt’ have been reported to cause bromoderma.
Other sources of bromine have included the pesticide methyl bromide, brominated spa pool disinfectants, flame retardants, permanent hair wave solutions and silver bromide used in photographic films/papers.
Iodine and iodides
Iodine is used topically, orally and by injection.
Iodine is commonly used in topical antiseptics and there have been rare reports of iododerma following prolonged use of iodine-containing antiseptics applied to large areas of broken skin, e.g., to burns and following surgery where the wound has been left open to heal.
Oral iodine is used in the treatment of some thyroid diseases, and potassium iodide for the skin diseases erythema nodosum and sporotrichosis. It is found in some expectorants, multivitamins and tonics.
Amiodarone, a medication used to treat angina and heart arrhythmias (palpitations), has rarely been reported to cause iododerma after 18-24 months use. Iodine may also be ingested in high-iodine containing foods such as seaweed, seafood and iodised salt, with rare cases of iododerma reported following prolonged and excessive ingestion.
Iodine is used as a radiocontrast medium for x-rays including CT scan, cholecystogram and pyelogram, either orally or by injection into the bloodstream. However most of the iodine administered this way would usually be excreted by the kidneys within 24 hours of administration.
The amount of iodine required to cause an iododerma reaction is variable.
Accumulation of the halogen in the body seems to be required for this reaction so it usually occurs with: –
prolonged or excessive use, and/or
acute or chronic kidney failure, as halogens are excreted from the body by the kidneys
A possible association has been suggested between halogenoderma and polyarteritis nodosa or myeloproliferative disorders such as multiple myeloma.
Clinical features
Generally, it takes months of continuous exposure to a halogen in order to develop a halogenoderma. However, in some cases it can be acute within days, particularly following administration of iodine-containing radiocontrast medium for x-rays in patients with kidney failure.
Lesions most commonly appear on the face and upper body, but involvement of the limbs and mucous membranes (mouth, conjunctiva of the eye) can also occur.
There are a number of clinical presentations of halogenodermas: –
Acne-like rash – pustules and small red lumps (acne medicamentosa)
Vegetating/fungating nodules – raised firm lumps
Exudative plaques – weepy, slightly raised, large areas
Vegetating or necrotic ulcer with pustules
Blisters – small or large; clear, pus- or blood-filled
Tuberous bromoderma – occurs mainly in infants and starts as small red bumps or pustules that rapidly merge into a raised plaque, most commonly on face, scalp and legs
Halogen panniculitis (inflammation of fat) – tender red swellings under the skin usually 1-2 cm in diameter, but can be up to 10 cm. Abscesses form that may ulcerate and scar. This is part of a systemic illness, initially with fever and diarrhoea, but with continued halogen exposure abdominal and muscle pain develop, as well as worsening of the initial symptoms.
More than one of these skin patterns may occur in an individual. Lesions such as ulcers and plaques may be solitary or multiple.
Swelling of the major salivary glands (parotid, submandibular) has been reported following iodine-containing contrast medium and has been called ‘iodine mumps’.
Rarely there may also be systemic symptoms and signs of halogen toxicity. Bromism includes muscle weakness, personality changes, abnormal walking gait and fits. Toxic effects of iodine include low blood pressure and slow heart rate, kidney failure, inflammation of small blood vessels (leukocytoclastic vasculitis), overactive thyroid and abnormal liver function.
How is the diagnosis made?
A diagnosis of halogenoderma is usually made on the basis of:
history of halogen exposure
clinical features
histopathology of a skin biopsy. Skin biopsy shows neutrophil white cells with eosinophils and lymphocytes in the epidermis forming small abscesses. There may also be some inflammation in the dermis and involving blood vessels. Bromoderma also tends to show thickening of the epidermis (pseudoepitheliomatous hyperplasia).
blood tests, which may confirm the high halogen level.
However none of these tests are diagnostic and it is the combination that makes the diagnosis.
Treatment
Stopping the halogen will generally result in resolution over 4-6 weeks. Bromide has a long half-life in the body, about 12 days, so it takes some time for the blood level to fall. The clinical improvement parallels the falling blood level of halogen.
Wound care may be required for ulcers.
Sometimes active treatment may also be required, including fluid tablets (e.g. frusemide or ethacrynic acid) or intravenous fluids to increase excretion of the halogen by the kidneys, and/or anti-inflammatory medications, such as topical or oral corticosteroids or oral ciclosporin.
Mild postinflammatory pigmentation or scarring may persist after the skin lesions have healed. Very rarely, halogenoderma may be fatal.
Proposed mechanisms
Several theories have been put forward to explain the development of halogenoderma.
Delayed hypersensitivity reaction/allergic reaction. Iodine binds to protein in the bloodstream and it is believed that an allergic reaction develops against this complex. Lymphocyte transformation tests with iodinated human serum albumin have been positive in some cases.
Excretion of halogen via sweat or oil glands may result in an inflammatory reaction in the skin.
Halogens may make usually non-pathogenic or harmless skin microorganisms pathogenic.
See also: http://emedicine.medscape.com/article/1090031-overview
This: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3754371/
Potassium iodide, as a saturated solution, is a valuable drug in the dermatologist's therapeutic arsenal and is useful for the treatment of different diseases due to its immunomodulatory features. However, its prescription has become increasingly less frequent in dermatology practice. Little knowledge about its exact mechanism of action, lack of interest from the pharmaceutical industry, the advent of new drugs, and the toxicity caused by the use of high doses of the drug are some possible explanations for that. Consequently, there are few scientific studies on the pharmacological aspects, dosage and efficacy of this drug. Also, there is no conventional standard on how to manipulate and prescribe the saturated solution of potassium iodide, which leads to unawareness of the exact amount of the salt being delivered in grams to patients. Considering that dosage is directly related to toxicity and the immunomodulatory features of this drug, it is essential to define the amount to be prescribed and to reduce it to a minimum effective dose in order to minimize the risks of intolerance and thus improve treatment adherence. This review is relevant due to the fact that the saturated solution of potassium iodide is often the only therapeutic choice available for the treatment of some infectious, inflammatory and immune-mediated dermatoses, no matter whether the reason is specific indication, failure of a previous therapy or cost-effectiveness.
This: http://www.ncbi.nlm.nih.gov/pubmed/7458376
Twenty-nine patients with erythema nodosum, nodular vasculitis, or erythema nodosum-like lesions associated with Behçet's syndrome were treated with potassium iodide. Administration of the drug for systemic effect showed a substantial effect in 11 of 15 patients with erythema nodosum, seven of ten with nodular vasculitis, and one of four with leg lesions of Behçet's syndrome. Relief of subjective symptoms, including tenderness, joint pain, and fever, occurred within 24 hours. Substantial improvement in the eruption occurred within a few days, and the lesions disappeared completely ten to 14 days after therapy was initiated. The patients to whom the medication was administered shortly after the initial onset of erythema nodosum seemed to respond most satisfactorily. The effect of the drug was marked in the patients with positive C-reactive protein reactions, joint pains, and/or fever. Possible mechanisms by which potassium iodide exerts its effect are discussed.
Here's a page about EN: http://www.medicinenet.com/erythema_nodosum/article.htm
And people telling their stories: http://www.medicinenet.com/erythema_nodosum/patient-comments-287-page2.htm
It's pretty horrible and it seems like what was said in one of the articles cited above might be the cause:
"Halogens may make usually non-pathogenic or harmless skin microorganisms pathogenic."
And not just in the skin, but throughout the body. When the iodine starts kicking that stuff out, apparently one can get quite sick in a number of ways. My guess is that it is not the iodine or potassium iodide making the person sick, but the displaced/detoxing of the bromides/fluorides. But that can be very problematical!
Perhaps the peeps who get some of these reactions should switch over to potassium iodide and back off the lugol's? They mention several times the "immunomodulatory features" of potassium iodide. Maybe it's the combination of potassium and iodine in the potassium iodide formulation that is key?

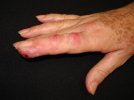
 )... Could it be that some critters are being activated by the iodine (as the Cs said) and, besides the detox symptoms, this could be due to a mycoplasma infection that "wakes up" with the lower doses? In that case, what would be the best approach to not wake the infection and, at the same time, not overwhelm the body with a "nuke" dose?
)... Could it be that some critters are being activated by the iodine (as the Cs said) and, besides the detox symptoms, this could be due to a mycoplasma infection that "wakes up" with the lower doses? In that case, what would be the best approach to not wake the infection and, at the same time, not overwhelm the body with a "nuke" dose?