From the videos, I think the protestors broke one of the rules of protesting. That is, do things in large numbers, where you are hard to neutralize. These guys were doomed from the start. There was only a couple of them, and they were in precarious positions on top of the trains. I found that to be very strange.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
The Politics of Climate Change: Green New Deal And Other Madness
- Thread starter Ennio
- Start date
Yozilla
The Living Force
Oh my, this is becoming crazy more and more. But what the people of Extinction Rebellion have in their minds? they don't need to go to work, evidently. They are so far away of the reality, the day by day, of people, workers, mothers that need to go to work, of families that have money difficulty, etc. They are a bunch of egocentric egoistical and individualist people. But you see, people start to see things with more clarity thanks to these idiots that are like the crazy prophets that sometimes are pictured on movies, remember, that announced the End of the World on the street. In fact they are clowns in this life that is a circus.
Maybe those XR dudes were Greenbaumed, BlueBummed or whatever - deeply programmed to be trigerred in times like this, to irritate public and force six-pack-Joes to to some kind of rebelion/upheaval...
Push there buttons, just push and push.... everyday and everyway
Another classical example (from the honest nest), of greed and manipulation.
A billionaire backer of Extinction Rebellion has been revealed to have a stake in Heathrow Airport.
Sir Chrisopher Hohn has clandestinely built a 4 per cent stake in Heathrow parent company Ferrovial worth €730m (£630m), according to the Sunday Telegraph.
It was reported in May by Spain’s Banco de Sabadell that Hohn had bought a one per cent share in the Spanish construction and services company.
It has now been revealed that Hohn has bought a further three per cent share in Ferrovial.
The billionaire hedge fund manager revealed this month he was the largest individual backer of Extinction Rebellion thanks to £200,000 in donations.
The activist group organised mass protests over the past two weeks, some of which were aimed at London’s airports.
Three flights at London City Airport were disrupted, after one passenger lied on top of a British Airways plane on the runway and another forced a flight to turn around shortly after take-off.
Protest action at Gatwick Airport was cancelled after a group of protesters were attacked by commuters at Canning Town Tube station.
Despite this, Hohn has a history of investments in airports.
He is one of three largest investors in Spanish company Aena – the majority owner of Luton Airport.
His investment company, TCI, is the second-biggest investor in Eurotunnel owner Getlink.
Speculation is also rife that Hohn may be looking to significantly increase his share in Ferrovial.
The company’s UK-based construction company Amey is involved in an ongoing spat with Birmingham Council over a £2.7bn highways contract.
Ferrovial is now looking to sell Amey, after it paid the council £215m to walk away from its contract.
Forty per cent of shares in Ferrovial are owned by the four sons of its late founder Rafael del Pino y Moreno.
A source told the Sunday Telegraph that the sons are acting separately in managing their respective stakes in the company.
They said: “In a go-private situation, a buyer does not need to win over each sibiling, whereas in the past, a buyer effectively required the support of the founding father.”
Ferrovial were contacted for comment and TCI declined to comment.
A billionaire backer of Extinction Rebellion has been revealed to have a stake in Heathrow Airport.
Sir Chrisopher Hohn has clandestinely built a 4 per cent stake in Heathrow parent company Ferrovial worth €730m (£630m), according to the Sunday Telegraph.
It was reported in May by Spain’s Banco de Sabadell that Hohn had bought a one per cent share in the Spanish construction and services company.
It has now been revealed that Hohn has bought a further three per cent share in Ferrovial.
The billionaire hedge fund manager revealed this month he was the largest individual backer of Extinction Rebellion thanks to £200,000 in donations.
The activist group organised mass protests over the past two weeks, some of which were aimed at London’s airports.
Three flights at London City Airport were disrupted, after one passenger lied on top of a British Airways plane on the runway and another forced a flight to turn around shortly after take-off.
Protest action at Gatwick Airport was cancelled after a group of protesters were attacked by commuters at Canning Town Tube station.
Despite this, Hohn has a history of investments in airports.
He is one of three largest investors in Spanish company Aena – the majority owner of Luton Airport.
His investment company, TCI, is the second-biggest investor in Eurotunnel owner Getlink.
Speculation is also rife that Hohn may be looking to significantly increase his share in Ferrovial.
The company’s UK-based construction company Amey is involved in an ongoing spat with Birmingham Council over a £2.7bn highways contract.
Ferrovial is now looking to sell Amey, after it paid the council £215m to walk away from its contract.
Forty per cent of shares in Ferrovial are owned by the four sons of its late founder Rafael del Pino y Moreno.
A source told the Sunday Telegraph that the sons are acting separately in managing their respective stakes in the company.
They said: “In a go-private situation, a buyer does not need to win over each sibiling, whereas in the past, a buyer effectively required the support of the founding father.”
Ferrovial were contacted for comment and TCI declined to comment.
Last edited:
I don't think this has been mentioned yet: This is Part One of an investigation into Extension Rebellion and Gail Bradbrook. A thorough report in the establishment for our Orwellian world. The comments at the end attack the content of the piece. Dr Gail Marie Bradbrook: Compassionate Revolutionary… for hire? – News from Nowhere
Introducing Gail Bradbrook, professional activist
In order to understand an organisation or entity, one must first come to an understanding of its founders. Dr Gail Marie Bradbrook, along with Julian ‘Roger’ Hallam, appear to be the two primary instigators of the Extinction Rebellion (XR) movement
Gail epitomises the new generation of ‘professional activists’, having positioned herself at the epicentre of the revolving door between big business, government bureaucracies and establishment-friendly NGOs, campaign groups and charitable organisations, all of which increasingly function as the public face of international corporate and financial power.
Acting through proxy organisations permits these forces to obscure the fact that government policies are being swayed by corporate entities that fund entire networks of charities and non-governmental organisations to interact with government on their behalf.
As well as having the ear of government, many of the directors and trustees of Gail’s Citizens Online charity just so happen to be people whose companies stand to make an absolute killing from the 5G roll-out – which is covertly promoted by Extinction Rebellion, due to its coming under the auspices of the United Nations’ Agenda 2030 (the current rebranding of Agenda 21), which is most often referred to with deceptive simplicity, as ‘Sustainable Development’ [SD]. Indeed, the smart grid / 5G infrastructure roll-out and the United Nation’s ‘Sustainable Development Goals’ (SDG) are entirely inseparable.

Introducing Gail Bradbrook, professional activist
In order to understand an organisation or entity, one must first come to an understanding of its founders. Dr Gail Marie Bradbrook, along with Julian ‘Roger’ Hallam, appear to be the two primary instigators of the Extinction Rebellion (XR) movement
Gail epitomises the new generation of ‘professional activists’, having positioned herself at the epicentre of the revolving door between big business, government bureaucracies and establishment-friendly NGOs, campaign groups and charitable organisations, all of which increasingly function as the public face of international corporate and financial power.
Acting through proxy organisations permits these forces to obscure the fact that government policies are being swayed by corporate entities that fund entire networks of charities and non-governmental organisations to interact with government on their behalf.
As well as having the ear of government, many of the directors and trustees of Gail’s Citizens Online charity just so happen to be people whose companies stand to make an absolute killing from the 5G roll-out – which is covertly promoted by Extinction Rebellion, due to its coming under the auspices of the United Nations’ Agenda 2030 (the current rebranding of Agenda 21), which is most often referred to with deceptive simplicity, as ‘Sustainable Development’ [SD]. Indeed, the smart grid / 5G infrastructure roll-out and the United Nation’s ‘Sustainable Development Goals’ (SDG) are entirely inseparable.
Euronews, a loud speaker for selective, safe, no-fakenews, carried an article from NBC which like a preparation for a war in Iraq or Libya, moves ahead: More than 11,000 scientists issue fresh warning: Earth faces a climate emergency The difference if any is that the population growth in the World has to be managed through various programmes "The authors say family planning services and other social justice efforts that promote full gender equity should be enacted to help stabilize the world's population,"
Euronews also carried Italy introduces mandatory climate change lessons in schools
By Denise Chow with NBC News Tech and Science News• last updated: 05/11/2019 - 22:55
An international consortium of more than 11,000 scientists is backing a study with a dire warning: Earth is facing a climate emergency.
The new study of how human activities have impacted the planet over the past four decades declares that harmful greenhouse gas emissions are rapidly rising, that governments are making insufficient progress in tackling the crisis, and that scientists have "a moral obligation to clearly warn humanity of any catastrophic threat." The findings were published Tuesday in the journal BioScience.
The research, led by ecologists William Ripple and Christopher Wolf at Oregon State University, identifies six key areas in which governments, businesses and members of the public can make critical changes, including addressing the planet's swelling population, which has been a contentious topic in the climate debate.
The authors say family planning services and other social justice efforts that promote full gender equity should be enacted to help stabilize the world's population, which is increasing by approximately 80 million people per year.
"Lots of scientists have steered away from talking about population because it's controversial," said Steve Easterbrook, director of the University of Toronto's School of the Environment, who was one of the study's signatories. "The policy recommendations the study makes about gender equity and making family planning available to bring down the birth rate — these are completely consistent with studies of what we need to do in response to climate change. I'm glad to see it given more prominence than it normally gets."
The study says countries should replace fossil fuels with renewable energy sources while also investing in technologies to extract carbon dioxide from the atmosphere. Governments should also end subsidies to fossil fuel companies and wealthier countries should support poorer nations in transitioning to cleaner energy sources.
In addition, nations need to sharply reduce emissions of potent pollutants such as methane, soot and hydrofluorocarbons, which are human-made compounds that are commonly used in air conditioning, refrigeration and aerosols, the study finds. The researchers say that reducing these short-lived pollutants could slow the planet's short-term warming trend by more than 50 percent over the next few decades.
Climate change mitigation efforts should focus on protecting and restoring ecosystems such as forests, coral reefs, savannas and wetlands, which naturally absorb and store carbon dioxide from the atmosphere, they added.
The study also says people should eat mostly plant-based food, which will improve health and lower greenhouse gas emissions from livestock, and economies should prioritize carbon-free initiatives and sustaining ecosystems, rather than focusing on GDP growth and the pursuit of affluence.
The study is based on 40 years of data that show how human activities have affected the planet, including changes in fossil fuel consumption, carbon dioxide emissions, rates of deforestation and global surface temperatures. The authors warn that climate change is intensifying faster than most scientists predicted and is "threatening natural ecosystems and the fate of humanity."
Urgent action is needed, the researchers caution, "to avoid untold suffering due to the climate crisis."
The report's signatories include scientists from 153 countries, known together as the Alliance of World Scientists.
"I was concerned that we are now making the environment a political issue, and the environment should not be seen as a partisan issue," Leslie Duram, a professor of geography and environmental resources at Southern Illinois University in Carbondale, said of her motivation to endorse the study's findings. "I want us all to realize that we, as human beings and inhabitants of this planet, need to come together to take action to help preserve the environment."
The study does highlight some progress that has been made, such as a 373 percent increase in solar and wind energy consumption per decade since 2000. But the authors point out that in 2018, solar and wind energy use was still 28 times smaller than fossil fuel consumption.
The new study reiterates many of the same findings as seminal reports from the United Nations' Intergovernmental Panel on Climate Change, but does emphasize the need to address the planet's swelling population, which has been a contentious topic in the climate debate.
Easterbrook said that part of his motivation to sign the study was to support the recent youth-led movements calling for climate action.
"There have been plenty of people willing to criticize these kids that perhaps they don't understand the science, but it's increasingly clear that a lot of the youth leading these protests understand the science much better than any of us," he said. "It was important for us scientists to say: yes, the situation is that dire."
Euronews also carried Italy introduces mandatory climate change lessons in schools
Italy is to become the first country to make climate change lessons compulsory in schools, Education Minister Lorenzo Fioramonti announced.
When children return to class in September 2020 after their summer holidays, their annual curriculum will also include 33 hours — approximately one per school week — dedicated to climate change and sustainable development. [...]
The following from good old George Carlin couldn‘t be more to the point at this day and age. Spot on:
Wow, thank you for that. Worked like a tonic.
Euronews, a loud speaker for selective, safe, no-fakenews, carried an article from NBC which like a preparation for a war in Iraq or Libya, moves ahead: More than 11,000 scientists issue fresh warning: Earth faces a climate emergency The difference if any is that the population growth in the World has to be managed through various programmes "The authors say family planning services and other social justice efforts that promote full gender equity should be enacted to help stabilize the world's population,"
Speaking of which, there seems to be a spate of articles making the rounds right now on the subject of population control aka "depopulation is good for the planet". So much is being promoted under the subverted banner of "being a good human for the planet" - that just amounts to induced feelings of guilt for being alive. This 'climate emergency' psyop covers so much ground, is so pervasive and covers so many areas of life that the controllers must be patting themselves on the back for their invention.
Its encouraging, however, to see that many have gotten wind of where all this may be heading:

We are Facing Depopulation, not Overpopulation - Activist Post
Contrary to what most people believe, this video concludes that we should be concerned about Depopulation instead of #Overpopulation.
End Of The American Dream
Life As You Have Known It Will Never Be The Same Again...
 endoftheamericandream.com
endoftheamericandream.com

From 7 Billion To 500 Million: This Is The Population Culling Agenda Of The Elite
The United Nations has officially designated October 31st, 2011 as “7 Billion Day”. On that day, the United Nations estimates that the population of the Earth hit 7 billion for the very…
It looks like many of what used to be viewed as separate manifestations of madness are converging into a paradigm of war on humanity, not only in the physical elimination sense but also in the somehow necessary mental slavery. Science fiction is too normal to describe our world nowadays.This 'climate emergency' psyop covers so much ground, is so pervasive and covers so many areas of life that the controllers must be patting themselves on the back for their invention.
It looks like many of what used to be viewed as separate manifestations of madness are converging into a paradigm of war on humanity, not only in the physical elimination sense but also in the somehow necessary mental slavery. Science fiction is too normal to describe our world nowadays.
Absolutely, and another forumite was saying pretty much the same thing in conversation this very morning. Science fiction can be so insightful sometimes:
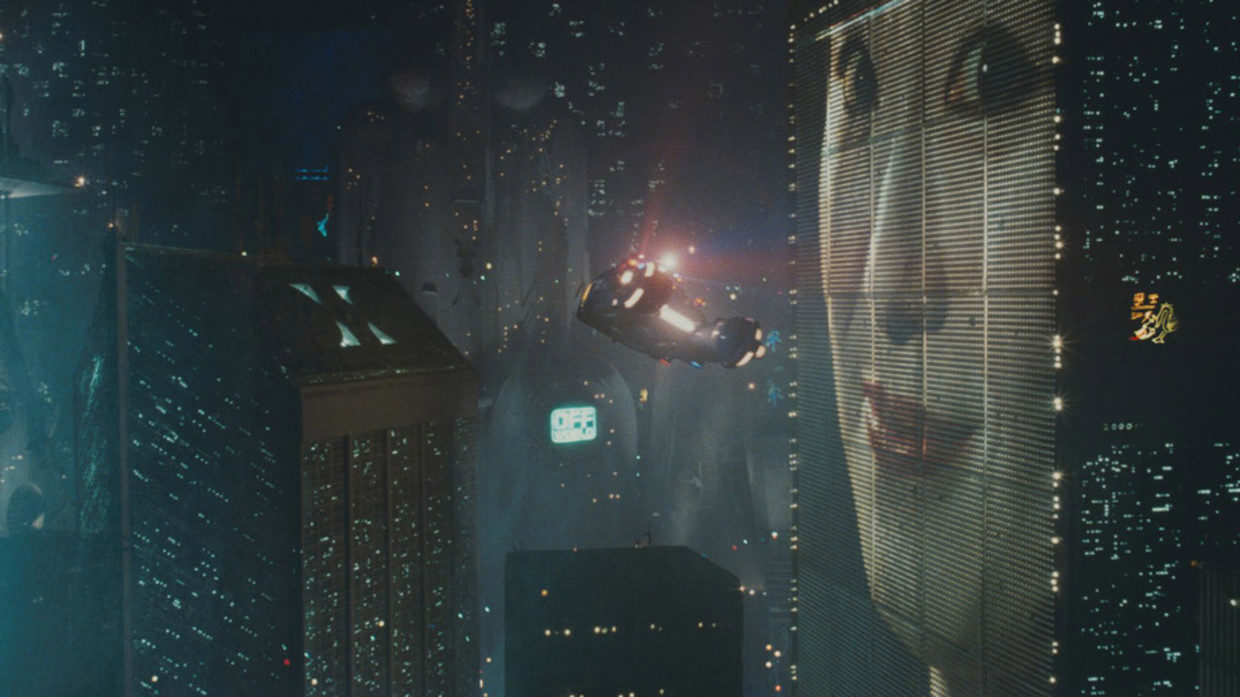
'Blade Runner' was set in November 2019: Instead of seeing it as a warning, we used it as an instruction manual
The real world has caught up to the dystopian sci-fi classic Blade Runner, set in November 2019. While robots aren't quite passing for humans, they're silently taking over human jobs and roles. The real Los Angeles of November 2019 bears more...
family planning services and other social justice efforts that promote full gender equity should be enacted to help stabilize the world's population,
So maybe some of those pushing the gender equality thing are doing so in order to 'empower' women so that they stop having children? Nice.
Speaking of which, there seems to be a spate of articles making the rounds right now on the subject of population control aka "depopulation is good for the planet".
It appears as a general call to reduce the population, but considering where the activism is being most loud are some more targeted than others? Is the next trend to be activated children that are convinced they can contribute to the survival of the planet if they remove their own ability to reproduce? Or worse, that they can save the planet, if they forcefully remove the ability of others?So maybe some of those pushing the gender equality thing are doing so in order to 'empower' women so that they stop having children? Nice.
As a comment to the first post earlier today, at the time, I was wondering if the articles were worth posting or commenting on. Now, considering the discussion and the comments, I wonder if I was almost put to sleep by the relentless stream of propaganda that bit by bit, day by day amounts to an effort to wear down resistance to the installation of self censorship and a way of thinking that conforms to the content and the views that are expected. It is actually scary when one thinks of the consequences.
Well, it really does seem like we have a ‘climate emergency.” The weather year by year does look to being more and more extreme and some level of ice age coming down the pipe. But as we all know well, the climate changes are not human created, as in what humans are doing materially to influence it but is theorized to be connected and related more to how humanity acts toward spiritual reality and an objective view of reality. There just isn’t anything humanity can do to stop the changes in a physical way. What might ameliorate the worst of the climate and cosmic environment’s impacting the earth and humans, in terms of the spiritual side and viewing and acting on an objective view of reality, is the exact opposite of what is being pushed out and for the most part swallowed by the public. So that points to more and building cosmic and climate chaos ahead as both are fed by the chaos building within humanity at large.
Also, with what the last few posts in this thread point out it looks like dark times are ahead when depopulation is going mainstream and tied into all the other distortions of reality and operations being foisted on humanity.
Also, with what the last few posts in this thread point out it looks like dark times are ahead when depopulation is going mainstream and tied into all the other distortions of reality and operations being foisted on humanity.
This article showcases the retired scientist who has garnered a LOT of followers. His basic message is "it's too late to do anything. Abrupt and catastrophic warming is happening and we are all going to die by 2026. He does write fluently and movingly and that seems to have swayed a lot of people. Problem is for my money he is in danger of founding a suicide cult.
Guy McPherson on Climate Change
Guy McPherson on Climate Change
Collective madness as a result of weather out of the normal has happened before. The following video
Dr. Baliunas on Weather Cooking from Climate Always Changes | Climate Change 101 explains a couple of instances during the period of the little ice age from 1550 to 1700 where the weather was extreme and on one of those occasions it resulted in hysteria..
First, on August 3rd 1562, a front with thunderstorms several hundred kilometers long struck Central Europe, it raged for a few hours which was followed by hail that lasted until midnight. The hail was so severe it killed birds, unprotected horses and cows, it destroyed vineyards and crops. 74 years later in late Spring on May 26, 1626 one meter of hail fell over parts of Germany followed by a severe frost that froze rivers, made grapevine plants explode, killed off the barley and wheat and denuded the trees. As a result of this unseasonal frost there came a demand to burn withches, they were accused of weather cooking with many thousands of people killed in parts of Germany.
Dr. Baliunas on Weather Cooking from Climate Always Changes | Climate Change 101 explains a couple of instances during the period of the little ice age from 1550 to 1700 where the weather was extreme and on one of those occasions it resulted in hysteria..
First, on August 3rd 1562, a front with thunderstorms several hundred kilometers long struck Central Europe, it raged for a few hours which was followed by hail that lasted until midnight. The hail was so severe it killed birds, unprotected horses and cows, it destroyed vineyards and crops. 74 years later in late Spring on May 26, 1626 one meter of hail fell over parts of Germany followed by a severe frost that froze rivers, made grapevine plants explode, killed off the barley and wheat and denuded the trees. As a result of this unseasonal frost there came a demand to burn withches, they were accused of weather cooking with many thousands of people killed in parts of Germany.
James A. Marusek has catalogued some 14,000 extreme weather events covering the years from 1 A.D. to 1900 A.D.
But for some reason, people have short memories, or perhaps weather cycles are slightly longer than a generation. Whatever the case, people often say “there had not been such weather in 100 years” – as Dr. Sallie Baliunas recounts in this video on “Weather Cooking.”
“Weather Cooking” was a crime that witches were accused of in the Medieval and Little Ice Age periods. When the weather got bad – it was clear to the ‘locals’ that someone had been up to something.

So – witches were executed for ‘cooking’ bad weather. (No wonder weathermen and weather women always look uncomfortable when their predictions of a nice day turned to rain!)
There are lots of comparisons as well by numerous authors about how the ‘green’ movement has adopted many of the more extreme religious overtones of that period of the hysterical past…when people were executed for cooking the weather. Of course they likely denied it. And that was proof they were guilty.
A response to The Ice Age Cometh! Forget Global Warming! actually another thread, but this comment became much more than a talk about ice age, as it deals with some of the arguments about CO2 and its behaviour in the geophysical system:
Piers Corbyn explains why reducing CO2 is not going to help by referring to Henry's Law. In this post there will be much more about Henry's Law, much more. I thought it would be a simple question, but it wasn't because in the process of understanding the small problem others kept coming. Before getting lost in the details, that may be relevant in some situations, Corbyn's argues that pumping CO2 out of the atmosphere will not help, because there is much more in the sea that then will be released. I think he is right, but others might say that CO2 appears faster in the atmosphere than it can be absorbed in the short term, and if they then believe that CO2 is all the fault of humans and the whole AGW ideology, then they think they have to do something.
Explanation of Henry's Law
If you like video there is a general explanation on Khan Academy, but it takes a few minutes: Henry's law and an example showing the difference in solubility between oxygen and carbon dioxide:

 www.khanacademy.org
www.khanacademy.org
There is also this explanation Henry's law - Wikipedia in text:

 scripps.ucsd.edu
scripps.ucsd.edu
View attachment 32462
The interpretation, I have is that the deviation of CO2 from being an ideal gas is more pronounced when it is at lower temperatures, when it is a pure CO2 gas, and less when it is mixed up with air. One possible explanation for this variation could be that CO2 is a more heavy molecule than both nitrogen (CO2 is three times heavier) and oxygen (1/3 heavier). At the same time, reading the numbers in the diagramme, this deviation from behaving as expected for an ideal gas is small, like less than one percent. This is important when one needs to get everything right, but I can still not find a clear explanation for the statement they have that the ocean absorbs relatively less now than at the beginning of the industrial revolution. To me a better explanation might be inertia, as there is only so much water surface compared to the kilometers of atmosphere above the ocean. Building high chimneys might reduce the time it takes to get close to water. Also there are more factories away from the sea and in hot climates and not cold Britain, where CO2 is less readily absorbed by the water. Maybe one could counter such arguments by claiming that the proximity to water is not so important if much of the CO2 that gets absorbed by the ocean actually does so indirectly by receiving rain that has absorbed CO2 in the atmosphere. However I don't know which plays a greater role. In the following I will work with Henry's law assuming that CO2 is pretty close to behaving as a natural gas.
Henry's Law and some calculations
The reason a gas exerts pressure is because the gas molecules move. The more they move, or the more that are confined to a unit of space the higher the pressure. If we from outside wish to change the speed of the many tiny gas molecules, we can change the temperature. If it is warmer, the molecules mover faster and the pressure increases, if it is colder they move slower, and the pressure of the gas decreases. This relationship is expressed in the ideal gas law:

 en.wikipedia.org
en.wikipedia.org
One mole of gas is the mass of 6.022 x 10^23 molecules. If we take air, it consists of various gases including nitrogen, oxygen, argon and others which make up about 0.4 % including water vapor and carbon dioxide. Each gas contributes to the pressure we experience in an approximate proportion to how much volume they take op.
There are tables that show how much water is needed to dissolve one mole of the various gases. In order for one mole of nitrogen to be dissolved in water at 25 degrees or 298 Kelvin at one atmosphere of pressure, one needs 1639 liters of water, for one mole of oxygen one needs 769 liters, but for CO2, carbon dioxide, one only needs 29.4 liters of water. One liter water is also 55,55 moles of H2O since one mole of water is 18 grams (The two moles of hydrogen atoms weigh 1 gram each and one mole of oxygen 16 grams, we combine them and get 1 mole of H2O, weighing 18 grams. 18 grams per mole times 55,55 moles per liter gives us 1000 grams per liter). Therefore in some tables they will tell you that in order to dissolve one mole of CO2 at one atmosphere you will need around 1630 moles of water [1630 atm · mol soln / molgas]. (This is approximately 55.55 moles of per liter of water times 29.4 atm liter of water/moles of CO2) The different methods of listing the Henry constants are explained both in the Wiki and in this document: Use Henry's Law to Calculate Concentration of Gas in a Solution
The amount that can be dissolved varies very much with the temperature, as I alluded to previously when I mentioned factories in hot climates versus cold climates. At a lower temperature more CO2 can be dissolved in water. From the diagram below, it appears that cold water at 0 degrees can hold twice as much CO2 as warm water at 25 degrees when the pressure from CO2 in the gas above the water is the same. https://demonstrations.wolfram.com/TemperatureDependenceOfHenrysLawConstant/
View attachment 32459
The units along the y-axis are different and not described, but the idea is, as mentioned, that at one atmosphere of pressure one needs 1630 moles of water to dissolve on mole of CO2.
If we compare the solubility of oxygen with that of carbon dioxide at 25 degrees, then we have 769 liters/ ("/" is divided by) 29.4 liters which gives us that carbon dioxide is 26 times more soluble in water than oxygen, at least when the temperature is 25 degrees.
In the air, there are by volume 20.95 % of oxygen according to Air - Molecular Weight and Composition while CO2 is around 410 ppm (parts per million) according to Climate Change: Atmospheric Carbon Dioxide | NOAA Climate.gov 410 ppm is 410/1000,000 or 0.041 %. If one compares the volume percentage of oxygen with that of CO2, that is 20.95 % / 0.041 % then we have that the volume amount of oxygen, O2 is about 510 times larger than the volume amount of carbon dioxide, CO2.
If we compare the results in two previous paragraphs, then on the one hand we found that CO2 is 26 times more soluble in water than oxygen. We also found that oxygen, in the air we breathe by volume takes up 510 more space than CO2. If this is true then we should find 510/26 or about 20 times more oxygen than CO2 dissolved in the water.
The ICCP in their report have thought about storing CO2 in the deep ocean. https://www.ipcc.ch/site/assets/uploads/2018/03/srccs_chapter6-1.pdf there is this illustration:View attachment 32467
Since the pressure increases rapidly with depth, (one atmosphere for every 10,33 meters), then the ocean could contain more CO2, just like a bottle of sparling water contains more than the usual water.
See also Ocean storage of carbon dioxide - Wikipedia
These people wish to vilify CO2, so essential for life, rather than allowing it to find a place where it wants, and have a chance to get a life, quite literally, as say carbon in a plant, oxygen for breathing or? Most of what they wish to bury is not carbon, it is oxygen. If one mole of CO2 is 44 grams, the 32 are from oxygen, only 12 from carbon. Isn't this all sadly symbolic?
For something more uplifting consider this shell model of carbon dioxide from Shell Model of Carbon Dioxide

And why not present the story, from the same page, behind the model which tells us something about cooperation at a very fundamental level, an expression of deeper principles.
From the transcript fo the YoutubeFor those who might have missed it on Sott, astrophysicist Piers Corbyn (brother of Jeremy) lays it all out:
[...]
02:28 all 02:30 all sides will agree this that there is 02:32 50 times more carbon dioxide in the sea 02:36 than there is in the air 02:39 okay now that means and there's going to 02:43 be a level between them and the level 02:45 the balance level between the two of 02:47 them is the saturation level of co2 in 02:51 the air will depend on the temperature 02:53 of the sea you warm up the sea a bit 02:55 like warm up a glass of water some gases 02:57 will come off nitrogen oxygen carbon 02:59 dioxide will come off you cool down a 03:01 glass of water and it can absorb more of 03:04 those gases that's very straightforward 03:06 very simple and very basic physics 03:08 because there's 50 times more co2 in the 03:12 sea than in the air it means basically 03:14 whatever man does to the air will have 03:17 no effect because the co2 will 03:18 compensate if man puts extra co2 into 03:22 the air or nature or termites or anybody 03:25 put co2 into the air it will just go 03:27 into the sea depending on the Seas 03:29 temperature and if you take it out of 03:32 the air then it will come out of the sea 03:34 and the levels will stay the same 03:36 according to what's called Henry's law 03:39 issue the equilibrium levels of a liquid 03:43 and a gas at a certain temperature so 03:46 the co2 theory is wrong from the start 03:51 even if you believe a co2 is having an
03:54 impact the co2 levels can't change under 03:57 man's influence is a fact is the Sun 04:00 rules the sea temperature and the sea 04:03 temperature rules the climate
Piers Corbyn explains why reducing CO2 is not going to help by referring to Henry's Law. In this post there will be much more about Henry's Law, much more. I thought it would be a simple question, but it wasn't because in the process of understanding the small problem others kept coming. Before getting lost in the details, that may be relevant in some situations, Corbyn's argues that pumping CO2 out of the atmosphere will not help, because there is much more in the sea that then will be released. I think he is right, but others might say that CO2 appears faster in the atmosphere than it can be absorbed in the short term, and if they then believe that CO2 is all the fault of humans and the whole AGW ideology, then they think they have to do something.
Explanation of Henry's Law
If you like video there is a general explanation on Khan Academy, but it takes a few minutes: Henry's law and an example showing the difference in solubility between oxygen and carbon dioxide:

O2 and CO2 solubility (video) | Gas exchange | Khan Academy
Get an intuition for why carbon dioxide is so much more soluble than oxygen when it goes into water. Rishi is a pediatric infectious disease physician and works at Khan Academy. These videos do not provide medical advice and are for informational purposes only. The videos are not intended to...
There is also this explanation Henry's law - Wikipedia in text:
I am going to come back to Henry's Law but just one observation, because it seems not all scientists would use it as part of an explanation or maybe the journalist mixed it up:In physical chemistry, Henry's law is a gas law that states that the amount of dissolved gas in a liquid is proportional to its partial pressure above the liquid. The proportionality factor is called Henry's law constant. It was formulated by the English chemist William Henry, who studied the topic in the early 19th century. In his publication about the number of gases absorbed by water,[1] he described the results of his experiments:
..."water takes up, of gas condensed by one, two, or more additional atmospheres, a quantity which, ordinarily compressed, would be equal to twice, thrice, &c. the volume absorbed under the common pressure of the atmosphere."
An example where Henry's law is at play is in the depth-dependent dissolution of oxygen and nitrogen in the blood of underwater divers that changes during decompression, leading to decompression sickness. An everyday example is given by one's experience with carbonated soft drinks, which contain dissolved carbon dioxide. Before opening, the gas above the drink in its container is almost pure carbon dioxide, at a pressure higher than atmospheric pressure. After the bottle is opened, this gas escapes, moving the partial pressure of carbon dioxide above the liquid to be much lower, resulting in degassing as the dissolved carbon dioxide comes out of solution.

How Much CO2 Can The Oceans Take Up?
A companion phenomenon of emitting CO2 into the atmosphere is the loading of the oceans with elevated levels of carbon dioxide created by fossil fuel burning and other human activities. Recent estimates have calculated that 26 percent of all the carbon released as CO2 from fossil fuel burning, ce
I have some difficulty understanding how the above makes sense according to Henry's law, if there is an equilibrium between the amount in the gas and the amount in the water, a higher concentration of CO2 in the atmosphere should alow for more CO2 to be absorbed by the water, but is he suggesting it is not as proportionate as one should expect? There is a slide show https://www.iaea.org/sites/default/files/18/07/oa-chemistry-dickson-050916.pdf with some more ocean chemistry. From the above there was a recommendation to consult the Guide to Best Practices for Ocean CO2 Measurements There they introduce a concept, fugacity, that revolve around the reality that CO2 does not behave completely like an ideal gas. The explanation in one chapter is followed by another filled with chemistry and mathematics. If one takes a look at one illustration from page 3 of chapter 2 there is:But will the oceans always be able to take up that proportion of human CO2 emissions year in and year out?
Probably not in the near term, said Scripps Institution of Oceanography, UC San Diego marine chemist Andrew Dickson.
[...]
Dickson noted that although the oceans presently take up about one-fourth of the excess CO2 human activities put into the air, that fraction was significantly larger at the beginning of the Industrial Revolution. That’s for a number of reasons, starting with the simple one that as one dissolves CO2 into a given volume of seawater, there is a growing resistance to adding still more CO2.
View attachment 32462
The interpretation, I have is that the deviation of CO2 from being an ideal gas is more pronounced when it is at lower temperatures, when it is a pure CO2 gas, and less when it is mixed up with air. One possible explanation for this variation could be that CO2 is a more heavy molecule than both nitrogen (CO2 is three times heavier) and oxygen (1/3 heavier). At the same time, reading the numbers in the diagramme, this deviation from behaving as expected for an ideal gas is small, like less than one percent. This is important when one needs to get everything right, but I can still not find a clear explanation for the statement they have that the ocean absorbs relatively less now than at the beginning of the industrial revolution. To me a better explanation might be inertia, as there is only so much water surface compared to the kilometers of atmosphere above the ocean. Building high chimneys might reduce the time it takes to get close to water. Also there are more factories away from the sea and in hot climates and not cold Britain, where CO2 is less readily absorbed by the water. Maybe one could counter such arguments by claiming that the proximity to water is not so important if much of the CO2 that gets absorbed by the ocean actually does so indirectly by receiving rain that has absorbed CO2 in the atmosphere. However I don't know which plays a greater role. In the following I will work with Henry's law assuming that CO2 is pretty close to behaving as a natural gas.
Henry's Law and some calculations
The reason a gas exerts pressure is because the gas molecules move. The more they move, or the more that are confined to a unit of space the higher the pressure. If we from outside wish to change the speed of the many tiny gas molecules, we can change the temperature. If it is warmer, the molecules mover faster and the pressure increases, if it is colder they move slower, and the pressure of the gas decreases. This relationship is expressed in the ideal gas law:

Ideal gas law - Wikipedia
Ideal gas law - WikipediaThe ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stated by Émile Clapeyron in 1834 as a combination of the empirical Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law.[1] The ideal gas law is often written as
{\displaystyle PV=nRT,}
where {\displaystyle P}, {\displaystyle V} and {\displaystyle T}
and {\displaystyle T} are the pressure, volume and temperature; {\displaystyle n}
are the pressure, volume and temperature; {\displaystyle n} is the number of moles of gas; and {\displaystyle R}
is the number of moles of gas; and {\displaystyle R} is the ideal gas constant. It is the same for all gases.Ideal gas law - Wikipedia
is the ideal gas constant. It is the same for all gases.Ideal gas law - Wikipedia
One mole of gas is the mass of 6.022 x 10^23 molecules. If we take air, it consists of various gases including nitrogen, oxygen, argon and others which make up about 0.4 % including water vapor and carbon dioxide. Each gas contributes to the pressure we experience in an approximate proportion to how much volume they take op.
There are tables that show how much water is needed to dissolve one mole of the various gases. In order for one mole of nitrogen to be dissolved in water at 25 degrees or 298 Kelvin at one atmosphere of pressure, one needs 1639 liters of water, for one mole of oxygen one needs 769 liters, but for CO2, carbon dioxide, one only needs 29.4 liters of water. One liter water is also 55,55 moles of H2O since one mole of water is 18 grams (The two moles of hydrogen atoms weigh 1 gram each and one mole of oxygen 16 grams, we combine them and get 1 mole of H2O, weighing 18 grams. 18 grams per mole times 55,55 moles per liter gives us 1000 grams per liter). Therefore in some tables they will tell you that in order to dissolve one mole of CO2 at one atmosphere you will need around 1630 moles of water [1630 atm · mol soln / molgas]. (This is approximately 55.55 moles of per liter of water times 29.4 atm liter of water/moles of CO2) The different methods of listing the Henry constants are explained both in the Wiki and in this document: Use Henry's Law to Calculate Concentration of Gas in a Solution
The amount that can be dissolved varies very much with the temperature, as I alluded to previously when I mentioned factories in hot climates versus cold climates. At a lower temperature more CO2 can be dissolved in water. From the diagram below, it appears that cold water at 0 degrees can hold twice as much CO2 as warm water at 25 degrees when the pressure from CO2 in the gas above the water is the same. https://demonstrations.wolfram.com/TemperatureDependenceOfHenrysLawConstant/
View attachment 32459
The units along the y-axis are different and not described, but the idea is, as mentioned, that at one atmosphere of pressure one needs 1630 moles of water to dissolve on mole of CO2.
If we compare the solubility of oxygen with that of carbon dioxide at 25 degrees, then we have 769 liters/ ("/" is divided by) 29.4 liters which gives us that carbon dioxide is 26 times more soluble in water than oxygen, at least when the temperature is 25 degrees.
In the air, there are by volume 20.95 % of oxygen according to Air - Molecular Weight and Composition while CO2 is around 410 ppm (parts per million) according to Climate Change: Atmospheric Carbon Dioxide | NOAA Climate.gov 410 ppm is 410/1000,000 or 0.041 %. If one compares the volume percentage of oxygen with that of CO2, that is 20.95 % / 0.041 % then we have that the volume amount of oxygen, O2 is about 510 times larger than the volume amount of carbon dioxide, CO2.
If we compare the results in two previous paragraphs, then on the one hand we found that CO2 is 26 times more soluble in water than oxygen. We also found that oxygen, in the air we breathe by volume takes up 510 more space than CO2. If this is true then we should find 510/26 or about 20 times more oxygen than CO2 dissolved in the water.
The ICCP in their report have thought about storing CO2 in the deep ocean. https://www.ipcc.ch/site/assets/uploads/2018/03/srccs_chapter6-1.pdf there is this illustration:View attachment 32467
Since the pressure increases rapidly with depth, (one atmosphere for every 10,33 meters), then the ocean could contain more CO2, just like a bottle of sparling water contains more than the usual water.
See also Ocean storage of carbon dioxide - Wikipedia
These people wish to vilify CO2, so essential for life, rather than allowing it to find a place where it wants, and have a chance to get a life, quite literally, as say carbon in a plant, oxygen for breathing or? Most of what they wish to bury is not carbon, it is oxygen. If one mole of CO2 is 44 grams, the 32 are from oxygen, only 12 from carbon. Isn't this all sadly symbolic?
For something more uplifting consider this shell model of carbon dioxide from Shell Model of Carbon Dioxide
And why not present the story, from the same page, behind the model which tells us something about cooperation at a very fundamental level, an expression of deeper principles.
Isn't it beautiful?The carbon atom (in the middle) has four electrons in its outer shell.
The two oxygen atoms each has six electrons in their outer shells.
To complete their shells (which all atoms want to do), every one of these atoms needs to have 8 electrons in its outer shell.
So the central carbon needs to gain 4 electrons, and each oxygen atom needs to gain 2.
Because of the cloud-like nature of the electron, it can be in several places at once. An electron can move round two atoms at the same time. If an atom shares one of its electrons with another atom, BOTH atoms gain an electron, so filling a hole in their outer shells. So atoms join together to share pairs of electrons.
The carbon shares two of its electrons with each oxygen, so each oxygen gains two electrons and hence gains a full outer shell. Each oxygen shares two of its electrons with the carbon, so the carbon gains four electrons, and so gets a full outer shell too.
Each pair of electrons is called a covalent bond. So the carbon atom has 4 covalent bonds, two with each oxygen atom. We call these DOUBLE BONDs.
Trending content
-
-
Thread 'Coronavirus Pandemic: Apocalypse Now! Or exaggerated scare story?'
- wanderingthomas
Replies: 30K -
-
