While people are busy with Color protests CONvid agenda is obviously continuing with less opposition.
Robert Kennedy Jr. has new info on convid vaccines. I wonder what Trump has to say about this because his government switched on "warp speed" to get these vaccines ready ASAP...
View attachment 37001
These vaccines include the
Johnson & Johnson vaccine and
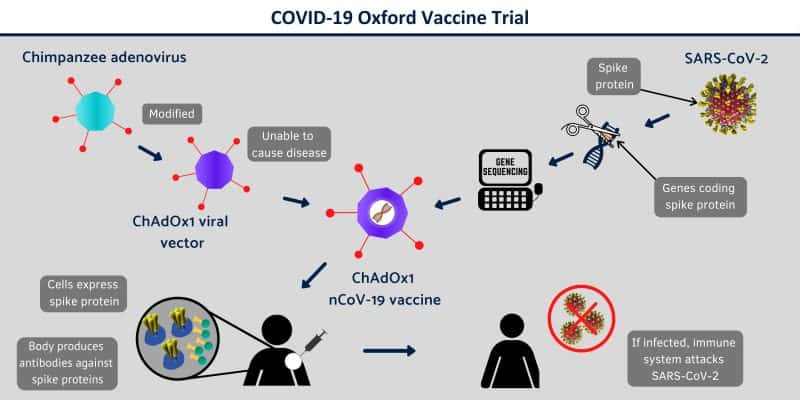
AstraZeneca’s “Oxford” vaccines which could be the first coronavirus vaccine available in the United States.
Both research groups are on the Trump administration’s “warp-speed” short-list for funding to develop a coronavirus vaccine by January 2021.
Other COVID vaccine candidates using cell lines from aborted babies
include CanSino Biologics, Inc. and the Beijing Institute of Biotechnology and the University of Pittsburgh according to a report published today in Science.
Some are using a cell line from a baby who was aborted in 1972, while others are using one from a baby aborted in 1985. These vaccines include DNA fragments from the infants with potential perils to human health. Dr Theresa Deisher PHD is one of the world’s leading researchers studying the impacts of injected fetal tissues on human health including cancers.
Regarding the CanSino Biologics, I discovered what I think may be some interesting information
Coronavirus study in China shows CanSino's vaccine spurs immune responses
From the article published on May 22
The
first person treated in that trial, which is being run by the National Institute of Allergy and Infectious Diseases, was vaccinated on March 16, around the same time that Chinese researchers nearly 6,000 miles away inoculated for the first a healthy adult in Wuhan with CanSino's shot.
Over the next 11 days, a total of 108 healthy adults were enrolled into CanSino's trial and selected to receive either a low, medium or high dose of the vaccine.
The Lancet data suggest that pre-existing immunity to the adenovirus used by CanSino could be interfering with the desired immune responses to vaccination. Fifty-five participants had high pre-existing immunity to CanSino's
chosen adenovirus upon enrolling in the study and, in those people, analysis showed that familiarity to the virus partially compromised the development of neutralizing antibodies.
Researchers also noted that older age "could have a negative effect on the vaccine-elicited responses to SARS-CoV-2." If borne out, that could pose a major hurdle to use of CanSino's vaccine among the older populations who are most at risk of severe COVID-19. The
engineered antibodies many other drugmakers are developing for the disease, by comparison, likely wouldn't have those issues, though the protection they'd offer is temporary,
In addition to that trial in China, CanSino
recently secured a go-ahead from Canadian health authorities to begin study of the vaccine there, as China's outbreak has now waned. CanSino is partnered with the Beijing Institute of Biotechnology on vaccine development.
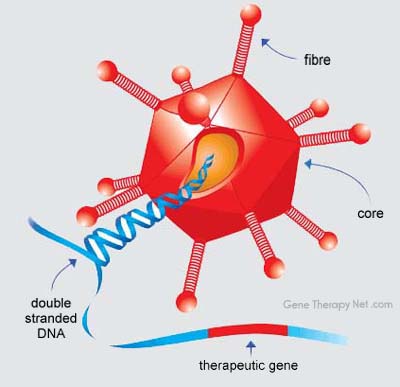
This from wikki regarding Adenoviruses

en.wikipedia.org
Adenoviruses (members of the
family Adenoviridae) are medium-sized (90–100
nm),
nonenveloped (without an outer lipid bilayer)
viruses with an
icosahedral nucleocapsid containing a double stranded
DNA genome. Their name derives from their initial isolation from human
adenoids in 1953.
[1]
They have a broad range of
vertebrate hosts; in humans, more than 50 distinct adenoviral
serotypes have been found to cause a wide range of
illnesses, from mild respiratory infections in young children (known as the
common cold)
to life-threatening multi-organ disease in people with a weakened immune system.
This does not look like a good match for those with comorbidities or immune system compromise.
From the CanSino webpage
Read our latest press releases, learn about new products, and stay up-to-date on how PNI is revolutionizing nanomedicine drug development globally.

www.precisionnanosystems.com
CanSinoBIO is at the forefront of COVID-19 vaccine development efforts with their Recombinant Novel Coronavirus Vaccine (Adenovirus Type 5 Vector) candidate (the Ad5-nCoV ) currently in phase II clinical trial in China. CanSinoBIO recently announced a collaboration with the National Research Council of Canada to initiate clinical testing of the Ad5-nCoV vaccine candidate in Canada. In parallel to clinical development of Ad5-nCoV, CanSinoBIO wants to join efforts with PNI to develop and commercialize a mRNA-LNP based vaccine. CanSinoBIO's co-founder, chairman and CEO Dr. Xuefeng Yu said, "Our team has been dedicated to developing safe and effective vaccines to fight against the COVID-19 pandemic. Since RNA vaccines are a disruptive technology as they do not require cell culture, utilize synthetic delivery and have a smaller manufacturing footprint, our partnership with PNI to advance a mRNA-LNP vaccine candidate will not only help accelerate the process, but will also potentially revolutionize the vaccine industry."
They also use nanotechnology in there products
Read our latest press releases, learn about new products, and stay up-to-date on how PNI is revolutionizing nanomedicine drug development globally.

www.precisionnanosystems.com
VANCOUVER, British Columbia, Canada – Precision NanoSystems, Inc. (PNI), a privately held biotechnology company developing proprietary microfluidic-based manufacturing technology for the use and development of cutting edge nanomedicines, today launched its commercial SUB9KITSTM reagents at the Society for Neuroscience (SFN) meeting. The Annual SFN meeting is the industry leading conference for molecular neuroscientists, attracting over 30,000 scientists and industry leaders.
PNI will be launching its Neuro9KIT, specifically designed for the study of genes in diseases of the brain. Additionally, PNI has been invited to present data on the Neuro9KIT at the conference in a talk entitled “ApoE-Enhanced Lipid Nanoparticle Delivery of siRNA Silences Neuronal Gene Expression in vitro & in vivo”. PNI will provide i
nterested customers demonstration Neuro9KITS that fluoresce when administered into neuronal cells, allowing users to rapidly assess the effectiveness of the kit in their given model of disease.
So this seems to mem that they will be will utilizing nanotechnology in this vaccine, could this also be used as a tracking technology that has been suggested by some researchers. I also wonder if there will be disclosure of adverse affects on vulnerable populations, if this vaccine becomes available to the general population, some children are also immune compromised.