While I was writing my last post,
Protecting Against Spike Protein Toxicity With Sulfur, Selenium, and Sunlight, I came across
this paper from 2002 showing that, in dopamine-responsive brain cells, vitamin C
worsens nitric oxide toxicity, while glutathione and N-acetyl-cysteine
protect against it.
The context of this study was an investigation into the damage of dopamine-responsive brain cells in the context of Parkinson’s.
However, I believe this paper demonstrates a much more broadly applicable general principle: high doses of vitamin C need to be properly balanced with glutathione support in any context where nitric oxide toxicity might be important. That includes COVID and vaccine-induced spike protein toxicity, and it should be broadly applicable to inflammation in general.
I am working on a much more extensive report tying this together with vitamin C research in the context of COVID, general immunity, and cancer. I will likely release this report at the end of next week.
For now, I wanted to leave you with my preliminary take-home points so that you can take them under consideration now if you use high-dose vitamin C for any purpose.
This post will remain available until the final report is out next week. At that point, it will be integrated into the final report.
Since the final report will be contributing to my upcoming Vaccine Guide as well as version 8 of the COVID Guide (the last update I will make before returning full-time to finishing my book), it will be available to everyone for the first 48 hours and become paid-only afterwards.
In Figure 7A, we see that in these dopamine-responsive brain cells, a nitric oxide donor (2nd column) increased the percent of cells that were dying compared to the control (1st column), and that neither 200 nor 400 micromoles per liter (μmol/L) of
ascorbic acid (vitamin C) had any effect alone (columns 3 and 4), but actually increased cell death in response to the nitric oxide donors (columns 5 and 6).
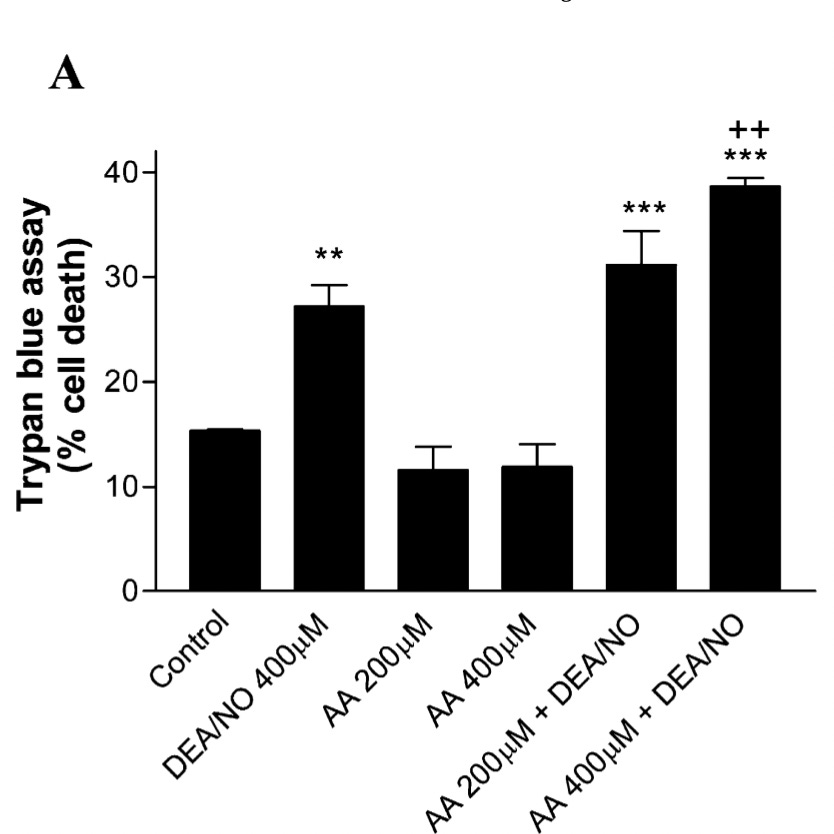
The asterisks indicate a statistically significant difference from the control, while the crosses indicate a statistically significant difference from the nitric oxide donor alone. So, we see that only 400 μmol/L produced a statistically significant worsening.
However, the mean cell death with 200 μmol/L is about 15% higher on a relative basis than with the nitric oxide donor alone. This suggests to me that the effect is beginning slightly under 200 μmol/L but becomes more meaningful after 400 μmol/L.
As seen in Figure 4A, by contrast, 600 μmol/L of either glutathione or N-acetyl-cysteine (NAC) completely abolishes the increase in cell death:
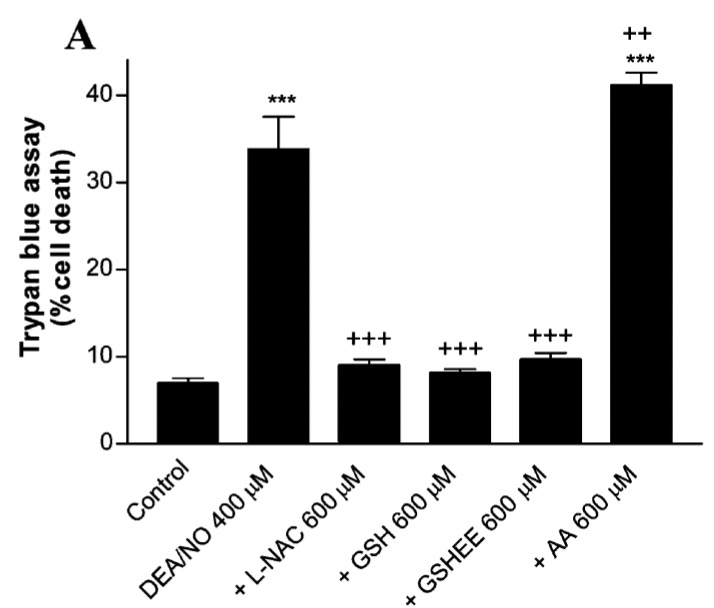
Overall, these results are consistent with the following explanation:
- As explained in my last post, glutathione prevents excessive S-nitrosylation of proteins, and a deficiency of glutathione can lead nitric oxide to engage in nitration of proteins. S-nitrosylation is a regulatory modification that occurs under inflammatory stress, and nitration may play a regulatory role but is often associated with irreversible damage to proteins.
- Thus, glutathione shifts nitric oxide away from inflammatory and damaging roles, and toward useful roles like vasodilation.
- Antioxidant protection occurs in a very defined order: vitamin E is recycled by vitamin C, vitamin C is recycled by glutathione, glutathione is recycled using energy from the pentose phosphate pathway using derivatives of niacin and riboflavin. This is a one-way street. Glutathione recycles vitamin C, but vitamin C does not recycle glutathione.
- To say “glutathione recycles vitamin C” means that vitamin C oxidizes glutathione. If vitamin C oxidizes glutathione faster than glutathione can be recycled itself, vitamin C will tax the glutathione pool and thereby render nitric oxide more likely to hurt than help.
Overall, this paper suggests that
vitamin C may begin to meaningfully tax the glutathione pool at concentrations close to 200 μmol/L and begins doing so in earnest by the time we arrive at concentrations of 400 μmol/L.
In my final report, I will integrate this with data on oral and intravenous vitamin C in humans. Suffice it to say for now that my review of that literature so far does not contradict using 200-400 μmol/L as benchmark plasma levels where we need to start getting concerned about balancing vitamin C with glutathione.
This paper provides a basis for translating this into oral and intravenous doses of vitamin C:
- A vitamin C-rich diet containing 300 mg per day will generally raise plasma levels to 70-85 μmol/L.
- Single doses of 1-2 grams will raise plasma levels to 150 to just shy of 200 μmol/L at the 5-hour mark and keep them around or above 100 μmol/L for about ten hours.
- Dosing 2.5 or 3 grams every four hours will keep plasma levels sustainably at or above 200 μmol/L.
- Intravenous doses of 3 or more grams consistently reach plasma levels well above 1000 μmol/L and 100 grams intravenously reaches over 15,000 μmol/L. Doses at or below 10 grams keep plasma levels elevated for about two hours, whereas 50 or 100 grams keep them elevated for 4-6 hours.
This study did not look at time points earlier than 5 hours, so it might be underestimating the peak concentrations reached.
The study also did not look at repeat dosing with less than 2.5 grams,
so it is quite possible that repeated dosing with 1 gram would also keep plasma levels at or above 200 μmol/L.
In the absence of a specific reason to use high-dose vitamin C, I think it is best to shoot for up to 400 milligrams per day of food-based vitamin C in at least two divided doses. Two studies (
here and
here) have shown that long-term consumption of 200 milligrams twice a day provides plasma and intracellular levels of vitamin C in the most beneficial range, with plasma levels in the high but sub-100 μmol/L range. They also show that this dose is well below the dose (1000 mg/d) required to raise urinary levels of urate and oxalate.
Here is the critical preliminary finding I wish to highlight now:
When using repeated doses of one gram or more, or long-term use of greater than 400 milligrams per day, for any concern relating to nitric oxide toxicity that includes anything inflammatory, it is critical to follow best practice for maintaining good glutathione status.
For your convenience, I have copied below the glutathione-specific recommendations from the
last post:
- Make sure to consume at least one gram of protein per kilogram of bodyweight or at least a half gram of protein per pound of bodyweight.
- Consider supplementing with 20-40 grams of whey protein, with anywhere from 500 mg each to 10 grams each of N-acetylcysteine and glycine, or anywhere from 500 mg to 5 grams of glutathione. My personal preference is for whey protein, supported by additional glutathione. I would stay toward 1500 mg of NAC or glutathione without a compelling reason to use higher doses, but consider what I’ve written here the top of the range to experiment with.
- Keep diabetes well treated, and reverse type 2 diabetes if possible, and reverse any signs of emerging pre-diabetes.
- Keep any thyroid, adrenal, or other metabolic disorder well treated.
- Eat a diet that keeps postprandial blood sugar under 140 mg/dL (7.7 mmol/L). Within that constraint, eat freely of whole fruit and unrefined sweeteners, but use moderation and steer very far away from 42% of Calories coming from simple sugar. Within these constraints, any increase in carbohydrate is likely to improve glutathione status by increasing insulin.
- Don’t start a new milk habit. If you use dairy products habitually, however, no need to stop.
- Manage your vitamin and mineral status properly, with special emphasis on selenium, thiamin, niacin, riboflavin, iron, magnesium, and calcium. =
When vitamin C is 400 milligrams per day or less, spread throughout meals or at least spread across two divided doses, it is unlikely to tax the glutathione pool. In fact, avoiding suboptimal vitamin C status is actually
good for the glutathione pool because it lowers the burden glutathione has to fulfill to keep it recycled.
It is still a good thing to support glutathione status.
However, it is at doses above this that spike plasma levels to 200 μmol/L or greater where robustly supporting glutathione status might make or break whether vitamin C helps or hurts.
At minimum, robustly supporting glutathione status will remove one of the concerns about vitamin C and shift it more clearly to the point of net benefit.





