Hepcidin
In 2001, a small antimicrobial peptide produced in the liver was identified and called hepcidin (from hep- “liver”, and -cidin from “microbicidal”). Hepcidin is cationic, amphipathic, and rich in cysteines, which generate four intramolecular disulfide bonds and stabilize the molecule in a folding structure β. Hepcidin is active against Gram-positive bacteria, but also inhibits the development of some fungi and Gram-negative bacteria with an antibacterial spectrum similar to that of β1-defensin. Nemeth et al. showed hepcidin production in response to interleukin-6. Other inflammatory cytokines also induced its synthesis, but to a lesser extent.
Hepcidin is the main regulator of iron homeostasis, because it binds to ferroportin, the only known iron transporter to the outside of the cell. Hepcidin binding to ferroportin induces the internalization and degradation of ferroportin, which blocks the exit of iron from the cells. The cells mainly affected by this interaction include enterocytes (which block iron absorption in the bowel) and macrophages (which prevent iron release into blood). The consequence is the occurrence of the so-called “anemia of chronic diseases”, due to decreased circulating iron concentrations.
Low serum iron levels restrict iron availability to extracellular bacteria, which are deprived of an essential nutrient and are therefore easier to kill. Paradoxically, however, intracellular pathogens (Salmonella, Mycobacteria, Candida, etc.) have greater amounts of iron available and may thus more easily develop inside macrophages, which therefore increase the local production of hepcidin to kill the germs.
Calcitriol has also been shown to modulate the iron–hepcidin–ferroportin axis. Bachetta et al. showed that the calcitriol-VDR complex may directly inhibit hepcidin expression by binding to a VDRE at the hepcidin promoter. On the other hand, calcitriol decreases interleukin-6 production in response to the lipopolysaccharide, and thus indirectly decreases IL-6 secretion. In healthy subjects and patients with kidney failure,
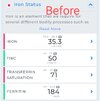
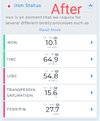
it has been shown that when 25-OH vitamin D levels increase, hepcidin decreases and anemia improves. Interestingly, a Spanish group had already shown that the intravenous administration of calcitriol to patients on hemodialysis improves anemia and reduces the need for erythropoietin.
Traditionally, calcitriol has been considered a calcium and phosphate regulating hormone,

www.elsevier.es