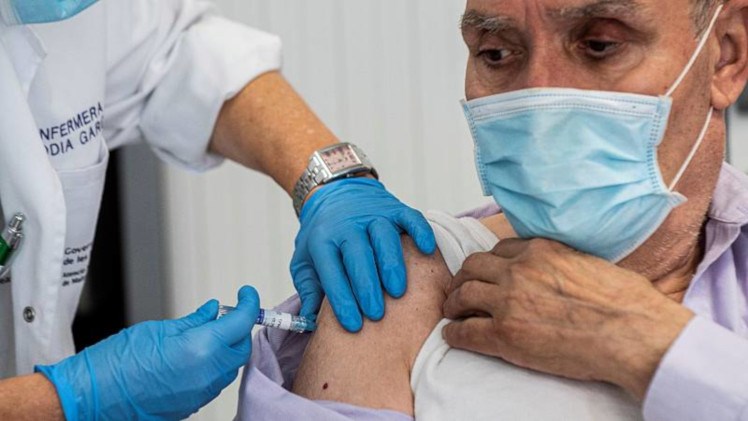
ICAN, through its attorneys, has written to HHS Secretary Alex Azar to demand that he use his authority under the National Childhood Vaccine Injury Act to mandate that all adverse events following COVID-19 vaccination be reported to the Vaccine Adverse Events Reporting System (VAERS) – a system co-administered by the CDC and FDA.
As discussed in our last legal update, the issue of underreporting to VAERS has been highlighted for over 30 years and is
still an ongoing problem. Many health care providers do not report to VAERS because they are not mandated to do so, they do not know what adverse events to look for or to connect to a vaccination, or because there is no routine follow-up with doctor or patient after an adverse event is reported. Until these very serious issues are corrected, and in order to provide the public with reliable information regarding the rate at which any given COVID-19 vaccine injury occurs, it is necessary that both manufacturers and health care providers be mandated to report any and all adverse events, aside from mild ones, to VAERS. This is precisely what ICAN, through its attorneys, led by Aaron Siri, has demanded of Secretary Azar in
its recent letter to him.
As Secretary of Health and Human Services, Azar has the authority under the National Childhood Vaccine Injury Act to require health care providers and vaccine manufacturers to report adverse reactions, events, and contraindications following the receipt of a vaccine to VAERS.
ICAN therefore requested that Azar immediately make it mandatory that all adverse events following a COVID-19 vaccination, with the exception of mild events, be reported by vaccine manufacturers and all health care providers to VAERS.
As it stands now, only a very limited number of adverse events
must be reported according to statute, namely shoulder injuries or fainting within seven days of receiving the vaccine or any serious issue resulting from such shoulder injury or fainting. The
Pfizer emergency use authorization does require that health care providers report a limited list of serious adverse events, however this list is not exhaustive and would not capture all adverse events including, for example, Bell’s Palsy which was seen in the clinical trials. There is also no indication the requirement to report such events will apply once full FDA licensure is granted for this vaccine.
Especially where clinical trials for COVID-19 vaccines are not reviewing for all adverse reactions and where the companies selling these products have no liability for injuries they cause, the American public must be confident that safety surveillance is the highest priority with these vaccines. For this and the other reasons above, ICAN has demanded that detailed and thorough mandates regarding adverse event reporting be put into place.
ICAN will continue to take additional legal steps to hold HHS, FDA, and CDC accountable for vaccine safety. ICAN will never stop fighting for true informed consent and continues to build its case that the federal government is withholding or obstructing the information needed to give such consent.


 .
.
