By
Robert F. Kennedy, Jr.
Meryl Nass, M.D.
Buried in the fine print of Monday’s approval by the U.S. Food and Drug Administration of the Pfizer Comirnaty COVID vaccine are two critical facts that affect whether the vaccine can be mandated, and whether Pfizer can be held liable for injuries.
Monday, the U.S. Food and Drug Administration (FDA)
approved a
biologics license application for the Pfizer Comirnaty vaccine.
The press
reported that
vaccine mandates are now legal for military, healthcare workers, college students and employees in many industries. New York City Mayor Bill de Blasio has now
required the vaccine for all teachers and school staff. The
Pentagon is proceeding with its
mandate for all military service members.
But there are several bizarre aspects to the FDA approval that will prove confusing to those not familiar with the pervasiveness of the FDA’s regulatory capture, or the depths of the agency’s cynicism.
First, the FDA acknowledges that while
Pfizer has “insufficient stocks” of the newly licensed Comirnaty vaccine available, there is “a significant amount” of the Pfizer-BioNTech COVID vaccine — produced under
Emergency Use Authorization (EUA) — still available for use.
The FDA
decrees that the Pfizer-BioNTech vaccine under the EUA should remain unlicensed but can be used “interchangeably” (
page 2, footnote 8) with the newly licensed Comirnaty product.
Second, the FDA pointed out that the
licensed Pfizer Comirnaty vaccine and the existing, EUA Pfizer vaccine are
“legally distinct,” but proclaims that their differences do not “impact safety or effectiveness.”
There is a huge real-world difference between products approved under EUA compared with those the FDA has fully licensed.
EUA products are
experimental under U.S. law. Both the
Nuremberg Code and federal regulations provide that no one can force a human being to participate in this experiment. Under
21 U.S. Code Sec.360bbb-3(e)(1)(A)(ii)(III), “authorization for medical products for use in emergencies,” it is unlawful to deny someone a job or an education because they refuse to be an experimental subject. Instead, potential recipients have an absolute right to refuse EUA vaccines.
U.S. laws, however, permit employers and schools to require students and workers to take
licensed vaccines.
EUA-approved COVID vaccines have an extraordinary liability shield under the
2005 Public Readiness and Preparedness Act. Vaccine manufacturers, distributors, providers and government planners are immune from liability. The only way an injured party can sue is if he or she can prove willful misconduct, and if the U.S. government has also brought an enforcement action against the party for willful misconduct. No such lawsuit has ever succeeded.
The government has created an extremely stingy compensation program, the
Countermeasures Injury Compensation Program, to redress injuries from all EUA products. The program’s parsimonious administrators have compensated
under 4% of petitioners to date — and
not a single COVID vaccine injury — despite the fact that physicians, families and injured vaccine recipients have reported more than
600,000 COVID vaccine injuries.
At least for the moment, the Pfizer Comirnaty vaccine has no liability shield. Vials of the branded product, which say “Comirnaty” on the label, are subject to the same product liability laws as other U.S. products.
When the Centers for Disease Control and Prevention’s (CDC) Advisory Committee for Immunization Practices places a vaccine on the
mandatory schedule, a childhood vaccine benefits from a generous retinue of liability protections.
But licensed adult vaccines, including the new Comirnaty, do not enjoy any liability shield. Just as with Ford’s exploding Pinto, or
Monsanto’s herbicide Roundup, people injured by the Comirnaty vaccine could potentially sue for damages.
And because adults injured by the vaccine will be able to show that the manufacturer knew of the problems with the product, jury awards could be astronomical.
Pfizer is therefore unlikely to allow any American to take a Comirnaty vaccine until it can somehow arrange immunity for this product.
Given this background, the FDA’s acknowledgement in its approval letter that there are insufficient stocks of the licensed Comirnaty, but an abundant supply of the EUA Pfizer BioNTech jab, exposes the “approval” as
a cynical scheme to encourage businesses and schools to impose illegal jab mandates.
The FDA’s clear motivation is to enable Pfizer to quickly unload inventories of a vaccine that science and the Vaccine Adverse Events Reporting System have exposed as unreasonably dangerous, and that the Delta variant has rendered obsolete.
Americans, told that the Pfizer COVID vaccine is now licensed, will understandably assume COVID vaccine mandates are lawful. But only EUA-authorized vaccines, for which no one has any real liability, will be available during the next few weeks when many school mandate deadlines occur.
The FDA appears to be purposefully tricking American citizens into giving up their right to refuse an experimental product.
While the media has trumpeted that the FDA has approved COVID vaccines,
the FDA has not approved the Pfizer BioNTech vaccines, nor any COVID vaccines for the 12- to 15-year age group, nor any booster doses for anyone.
And the FDA has not licensed any Moderna vaccine, nor any vaccine from Johnson & Johnson — so the vast majority, if not all, of vaccines available in the U.S. remain unlicensed EUA products.
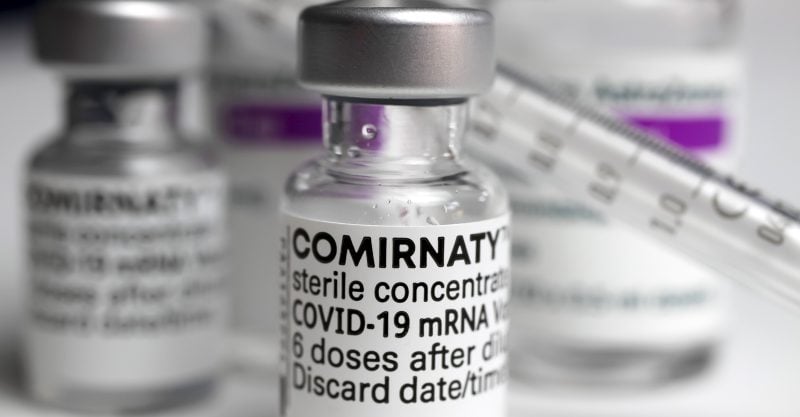
Here’s what you need to know when somebody orders you to get the vaccine: Ask to see the vial. If it says “Comirnaty,” it’s a licensed product.
If it says “Pfizer-BioNTech,” it’s an experimental product, and under 21 U.S. Code 360bbb, you have the right to refuse.
If it comes from Moderna or Johnson & Johnson (marketed as Janssen), you have the right to refuse.
The FDA is playing bait and switch with the American public — but we don’t have to play along.
If it doesn’t say Comirnaty, you have not been offered an approved vaccine.