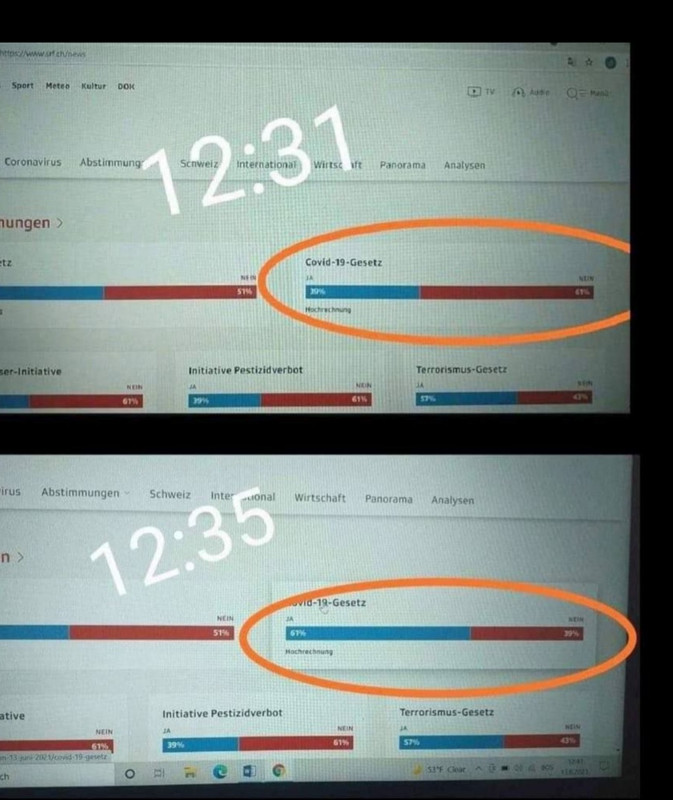
I think leftover ballots from the US election were shipped to Switzerland by Amazon. Despite all these travel restrictions, it's amazing how fast they have arrived when the health of the World Government was on the line!I'm not Swiss, unfortunately, but living in Swiss, since may 2021. It is very interesting in fact, this outcome of yesterdays referendum. People, who fight tis agenda have shared one interesting photo of results, which were publicly accessable, live time on web site. In just 4 minutes the results changed from 39% for the covid passes to 61% for covid passes. Don't understand how this is possible, or what happend in those 4 minutes?
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Coronavirus Pandemic: Apocalypse Now! Or exaggerated scare story?
- Thread starter wanderingthomas
- Start date
Natalija8
Jedi
Yes, he is the one, I wrote dr. Andreas Noack, thank you for shearing english version, Woodsman already shared from Odysee.come. Have no will to watch them two, because I watched the german twice, although it would be interesting, to compare, if they are the same.Hi Natalja,
I found this bitchute video, with english sub-titles, is it this one mentionned ?

MURDER! Just hours after publishing the research on the Vax, Dr Noack is DEAD.
🟢On this public and open discord server we are working things out and share our news and insights. 🟢If you feel like its time to connect and resist, feel free to join us: 🟢 https://app.revolt.chat/invite/XtpxNExv 🟢 This is Dr Noack, a chemist and graphene expert unlike any under in the EU. He...www.bitchute.com
Ocean
The Living Force
Hey presto! A magical new mutation out of thin air, …like it was just waiting to be released.
From http://peakprosperity.com:
Well, probably because the family tree for this variant makes zero evolutionary sense. At least to me. So far every subsequent mutation that has led to a new variant, or to a new family of variants (called a ‘clade’) has an easily tracked family history. Each new mutation is built upon the prior mutations.
If you plot these out with dots and line the resulting graph looks like a sideways shrub. Not with Omicron, or B.1.1.529. It sticks out like a whole different thing all on its own. I get concerned on a variety of levels when I see something so unusual, and I guess other people do too (but probably for very different reasons):
The way I read this ‘family tree’ it’s like someone reached all the way back to April 2020, plucked out an existing variant (having only the D614G mutation in common with every other clade & variant) and then somehow, magically, all by itself, came up with not one, not two, not ‘a few,’ but twenty new mutations never before seen in any other variant of concern.
From http://peakprosperity.com:
Well, probably because the family tree for this variant makes zero evolutionary sense. At least to me. So far every subsequent mutation that has led to a new variant, or to a new family of variants (called a ‘clade’) has an easily tracked family history. Each new mutation is built upon the prior mutations.
If you plot these out with dots and line the resulting graph looks like a sideways shrub. Not with Omicron, or B.1.1.529. It sticks out like a whole different thing all on its own. I get concerned on a variety of levels when I see something so unusual, and I guess other people do too (but probably for very different reasons):
The way I read this ‘family tree’ it’s like someone reached all the way back to April 2020, plucked out an existing variant (having only the D614G mutation in common with every other clade & variant) and then somehow, magically, all by itself, came up with not one, not two, not ‘a few,’ but twenty new mutations never before seen in any other variant of concern.
I've searched a little bit on the internet and found some information that Swiss Post is using software developed by Scytl, our known suspect (machine translated article):I'm not Swiss, unfortunately, but living in Swiss, since may 2021. It is very interesting in fact, this outcome of yesterdays referendum. People, who fight tis agenda have shared one interesting photo of results, which were publicly accessable, live time on web site. In just 4 minutes the results changed from 39% for the covid passes to 61% for covid passes. Don't understand how this is possible, or what happend in those 4 minutes?

Post kauft E-Voting-System und erntet dafür Kritik
Der spanische E-Voting-Anbieter Scytl hat Insolvenz angemeldet. Die Post kaufte sich zuvor die Rechte am Quellcode der Plattform – und will E-Voting in der Schweiz nun auf eigene Faust entwickeln.
I'm not Swiss, unfortunately, but living in Swiss, since may 2021. It is very interesting in fact, this outcome of yesterdays referendum. People, who fight tis agenda have shared one interesting photo of results, which were publicly accessable, live time on web site. In just 4 minutes the results changed from 39% for the covid passes to 61% for covid passes. Don't understand how this is possible, or what happend in those 4 minutes?
It would be great to have more evidence on this. What I noticed is that the screenshot on top cropped out the time at the bottom, while the lower screenshot does show it (12:41). And it is not exactly the time that the lower images claims it is (12:35), though close enough.
I've searched a little bit on the internet and found some information that Swiss Post is using software developed by Scytl, our known suspect (machine translated article):

Post kauft E-Voting-System und erntet dafür Kritik
Der spanische E-Voting-Anbieter Scytl hat Insolvenz angemeldet. Die Post kaufte sich zuvor die Rechte am Quellcode der Plattform – und will E-Voting in der Schweiz nun auf eigene Faust entwickeln.www-netzwoche-ch.translate.goog
It is interesting that Switzerland introduced this "e-voting software" just last year. This definitely points towards vote tampering, just like in the US election.
Last edited:
Hey presto! A magical new mutation out of thin air, …like it was just waiting to be released.
From http://peakprosperity.com:
Well, probably because the family tree for this variant makes zero evolutionary sense. At least to me. So far every subsequent mutation that has led to a new variant, or to a new family of variants (called a ‘clade’) has an easily tracked family history. Each new mutation is built upon the prior mutations.
If you plot these out with dots and line the resulting graph looks like a sideways shrub. Not with Omicron, or B.1.1.529. It sticks out like a whole different thing all on its own. I get concerned on a variety of levels when I see something so unusual, and I guess other people do too (but probably for very different reasons):
The way I read this ‘family tree’ it’s like someone reached all the way back to April 2020, plucked out an existing variant (having only the D614G mutation in common with every other clade & variant) and then somehow, magically, all by itself, came up with not one, not two, not ‘a few,’ but twenty new mutations never before seen in any other variant of concern.
With dozens of regularly expected mutations already reported, (with little fanfare), I have little doubt that this Omicron story is 99% a product of scripted public relations imagineering.
Ellis Medavoy, retired propagandist, in his landmark interview with Jon Rappoport, described inventing AIDS outbreaks in African villages out of thin air in much the same way.
Ocean
The Living Force
With dozens of regularly expected mutations already reported, (with little fanfare), I have little doubt that this Omicron story is 99% a product of scripted public relations imagineering.
Ellis Medavoy, retired propagandist, in his landmark interview with Jon Rappoport, described inventing AIDS outbreaks in African villages out of thin air in much the same way.
Completely agree.
And don't forget "Omicron", is an anagram for "moronic"
World will never eradicate Covid – Fauci
The White House’s chief medical adviser, Anthony Fauci, says it’s unlikely that the Covid-19 coronavirus will ever be wiped out, and insists the world is just going to have to start living with it.
It is way too early. I hope you feel better now, though I admit the video was sad and the deleted video disturbing, because while you found your second link, I also found the video reposted here: LaTika "DieFrontNews "I think, I will just lay down and give up.
Below I have tried to revisit graphene oxide, its properties, applications, and what could be done to eliminate it.I was watching this video from dr. Andreas Noack on friday, he was doctor of chemistry and he was specialist in development of new materials, he specialized him for carbon. He was talking in this video about watching research work from Profesor dr. Pablo Campra, from University of Almeira in Spain, with micro frame spectroskopy, examination of material on vibrational bands, were he found graphene oxide in vaccines.
Regarding the structure of graphene oxide, I was told about the following article, where one finds this illustration: Graphene and graphene oxide as nanomaterials for medicine and biology application June 2018 DOI:10.1007/s40097-018-0265-6 It shows graphene, graphene oxide and reduced graphene and their applications:
Among the above terms, ligands in biochemistry are explained in the Wiki as
It is encouraging that the likelihood of irreversible covalent bonding is small. It gives hope some changes could change back to what they were. The paper has an illustration showing the application of the graphene compounds:In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from ligare, which means 'to bind'. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion,[1] or protein[2] which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure. The instance of binding occurs over an infinitesimal range of time and space, so the rate constant is usually a very small number.
Binding occurs by intermolecular forces, such as ionic bonds, hydrogen bonds and Van der Waals forces. The association or docking is actually reversible through dissociation. Measurably irreversible covalent bonding between a ligand and target molecule is atypical in biological systems.
Andreas Noack explains Graphene Oxide (GO) as a razor. If that is so, why would people still react differently, are all GO compounds equally bad for the body, how long do GO impurities remain in the body, have they had problems with the different applications above, and what are the chances of detoxing from the influence of GO? Andreas Noack views the presence of GO in the vaccine from his perspective, but GO would also interact with other ingredients and the immune system of the body.
This video might indicate how the immune system could react. In Graphene Oxide Interactions with Innate Immune Cells..., one slide shows how the body may remove GO through the work of the neutrophils that in this example extends a net to capture a GO particle:
There may be more ways of eliminating the different varieties of graphene compounds, and especially aid the digestion of it. On this page, HOW TO REMOVE GRAPHENE OXIDE FROM THE BODY, they suggest that glutathione can help, and La Quinta Columa informs on more antioxidants that degrade graphene oxide including N-acetylcysteine, vitamin D, Milk Thistle, Melatonin, Zinc, and Quercetin. He also lists Astaxanthin, it is mentioned only once or twice in the protocol thread, but in many posts elsewhere on the forum.
About the fluorescent properties of graphene oxide
Less connected to the previous, but in the course of writing the post, I went back in the thread and watched: Pfizer Whistleblower Melissa McAtee on Vaccine Glowing And What Happened When She Confronted Management They mention the fluorescent properties of the Pfizer/BioTek vaccine. If GO is a suspect, there should be some papers, and there are. In Exploring the Origin of Blue and Ultraviolet Fluorescence in Graphene Oxide, Daichi Kozawa, Yuhei Miyauchi, Shinichiro Mouri, and Kazunari Matsuda.
The Journal of Physical Chemistry Letters 2013 4 (12), 2035-2040
DOI: 10.1021/jz400930f one finds:
I tried to match the color of the vials in the video with the 440 nm color. It is not too far away, but it is difficult to be sure. Another consideration is that there may be more than one fluorescent composite, even of GO. This study Fluorescent graphene oxide composites synthesis and its biocompatibility study discusses that GO with Cadmium, (Cd), which give a green fluorescence. If there is more than one colors, they would blend, just as a low concentration could make a color appear more like a hue.We studied the fluorescence (FL) properties of highly exfoliated graphene oxide (GO) in aqueous solution using continuous-wave and time-resolved FL spectroscopy. The FL spectra of highly exfoliated GO showed two distinct peaks at ∼440 (blue) and ∼300 nm [ultraviolet (UV)]. The FL of GO in the UV region at ∼300 nm was observed for the first time.
Regarding this story: Covid: Dutch police arrest quarantine hotel escapees
Dutch MSM published the following article:
Pretty insane! Poor people, I hope they can go to Spain soon.
Dutch MSM published the following article:
Carolina Pimenta from Spain was removed from a plane at Schiphol yesterday because she had tested positive for the corona virus after a flight from South Africa on Friday. She is now forced to stay with her partner Andrés in a hospital in Haren, near Groningen. "The suggestion that we escaped from quarantine is too ridiculous for words. Nobody told us what the rules are, we were treated like dogs."
Pimenta is a biomedical researcher. "I'd never think of doing something that would endanger the health of others. I know how important it is that everyone abides by the rules in this crisis. But we do need to know what those rules are exactly."
From pillar to post
She paints a shocking picture of what happened to her and her boyfriend when they wanted to transfer to a flight to Spain, their home, at Schiphol on Friday morning. Her story about the chaos at the airport and the way in which they have been sent from pillar to post since then, is in line with what other travelers say about the lack of reception and the faltering communication. “We were treated like dogs,” says Pimenta.
Before leaving South Africa, her PCR test was negative, as was her boyfriend's. After arriving in the Netherlands, she turned out to be one of 61 travelers whose new test was positive. She was taken to the hotel near Schiphol that was reserved for this group of passengers, insofar as they did not have a home in the Netherlands. Since they wanted to stay together, her boyfriend went with her.
False positive
"At the hotel, I asked the GGD [Dutch Municipal Health Service] if I could be retested, because I thought the test result could be false positive," says Pimenta. "No, they said: it is absolutely impossible for this test to give an incorrect result. Now, as a biomedical researcher, I know that it is very possible that a PCR test gives an unreliable result. My boyfriend tested negative while we were close to each other day and night – that was of course also remarkable."
The request for a retest was first rejected by the GGD, then granted, and finally failed. "The GGD employee in the hotel said that we should just buy self-tests, which we did. They both came out negative twice."
In the meantime, almost three days had passed since their arrival in the Netherlands. Their questions were not answered, they were left to their own devices, says Pimenta. "So yesterday I went to the GGD employee and the security guard downstairs in the hotel and said: I would like to have another PCR test, but it seems that no one is listening to us. What would you do? And they literally said: If we were in your position, we would just leave. Then I asked again just to be sure: can we get in trouble with that? No, they said."
'Like a criminal' disembarked
For their return flight to Spain, they were able to check in without any problems. Only in the plane was Pimenta's name called out and she was taken off board 'as if she was a criminal' by the military police 'with much fuss and screaming'. Her phone was then taken. Her boyfriend was also arrested when he came to inquire about what was going on.
First they were told that they had been arrested for evading the quarantine rules. But after the intervention of the Spanish ambassador, that charge appeared to have evaporated. "A police officer said: you can go, sorry for everything."
Yet they could not leave: at the direction of mayor Schuurmans of Haarlemmermeer, they were placed in quarantine again. People who 'endanger public health' may be required to go into isolation. The couple was taken to a hospital in Haren, more than a two-hour drive from Schiphol.
New test
"We saw a doctor for the first time this morning at 11 am. He responded very understandingly to our story. And half an hour later we finally got a new PCR test. We are now anxiously waiting for the results." What will happen next is still a mystery to her. "Can we go home if that new PCR test is negative? Will we still be prosecuted? We don't know."
The fact that the impression is given that they are two 'criminals' who 'wanted to flee' is what she finds the worst about the 'nightmare' they have been in for four days. That has never been the case, says Pimenta. "They play with our lives, with our good name, our freedom and our dignity."
'Left against the wishes of doctors'
Mayor Marianne Schuurmans of Haarlemmermeer says about the story of Carolina Pimenta and her partner to RTL Nieuws: "The GGD has told me that they left against the wishes of the doctors." Schuurmans says she has made inquiries with the police, the Marechaussee and the GGD. "They do not recognize what they've said."
Schuurmans says they "didn't want to stay in quarantine". Possible danger to public health was the main reason for the mayor to oblige them to go into isolation. This had to be done entirely in Haren, because elsewhere there was no place available that met the requirements set for isolation rooms.
Pretty insane! Poor people, I hope they can go to Spain soon.
Another paper showed up:About the fluorescent properties of graphene oxide
Less connected to the previous, but in the course of writing the post, I went back in the thread and watched: Pfizer Whistleblower Melissa McAtee on Vaccine Glowing And What Happened When She Confronted Management They mention the fluorescent properties of the Pfizer/BioTek vaccine. If GO is a suspect, there should be some papers,
In the paper, there is an image:Composition and Structure of Fluorescent Graphene Quantum Dots Generated by Enzymatic Degradation of Graphene Oxide
Xiaoyun He, Dan C. Sorescu, and Alexander Star
Cite this: J. Phys. Chem. C 2021, 125, 24, 13361–13369
Publication Date:June 15, 2021
https://doi.org/10.1021/acs.jpcc.1c01564
Copyright © 2021 American Chemical Society
Abstract
The wide applications of carbon nanomaterials (CNMs) in both materials and life sciences necessitate investigation of their metabolites due to the inevitable contact of CNMs and biological systems. Graphene oxide (GO), along with other types of CNMs, can be enzymatically degraded by myeloperoxidase (MPO), an enzyme released during the innate immune response. However, enzymatic degradation products are neither well-defined nor well-understood. Some products generated during MPO-catalyzed degradation of GO could emit blue photoluminescence (PL) and were simply dubbed graphene quantum dots (GQDs) without further elucidating their structures. In this work, we use liquid chromatography–mass spectrometry to isolate and elucidate chemical structures of the MPO-catalyzed degradation products. A general chemical formula screening workflow was developed for the GQDs, which are in the form of polyaromatic hydrocarbons (PAHs), obtained in the degradation products. Structures of the PAHs responsible for the blue PL were further proposed using density functional theory calculations. Our results indicated that structures with several conjugated benzene rings are likely to generate the observed PL. This work provides insights into the mechanism of enzymatic degradation and opens opportunities for fluorescence imaging of GO in biological systems.
That blue color is similar to the one in the video at minute 5.05
Is that a coincidence?
In the paper, one can go to table S4 and find that the fluorescence wavelength vary within the interval from 419 nm to 462 nm with a rough average somewhere in the middle. If the color they have chosen for their paper, reflects their results, then the comparison with the picture from the video is revealing.
Examples of compounds mentioned are:
And examples of their structures:
There are more details in the paper, if you are interested.
Natalija8
Jedi
It is way too early. I hope you feel better now, though I admit the video was sad and the deleted video disturbing, because while you found your second link, I also found the video reposted here: LaTika "DieFrontNews "
Below I have tried to revisit graphene oxide, its properties, applications, and what could be done to eliminate it.
Regarding the structure of graphene oxide, I was told about the following article, where one finds this illustration: Graphene and graphene oxide as nanomaterials for medicine and biology application June 2018 DOI:10.1007/s40097-018-0265-6 It shows graphene, graphene oxide and reduced graphene and their applications:
View attachment 51913
Among the above terms, ligands in biochemistry are explained in the Wiki as
It is encouraging that the likelihood of irreversible covalent bonding is small. It gives hope some changes could change back to what they were. The paper has an illustration showing the application of the graphene compounds:
View attachment 51921
Andreas Noack explains Graphene Oxide (GO) as a razor. If that is so, why would people still react differently, are all GO compounds equally bad for the body, how long do GO impurities remain in the body, have they had problems with the different applications above, and what are the chances of detoxing from the influence of GO? Andreas Noack views the presence of GO in the vaccine...
Thank you, @thorbiorn, great summary of dr. A. Noacks video on GO, which is a bit older, but in his last video, which has obviously cost him his live, he actualy argues the claim of profesor dr. Pablo Campra, University of Almeira, Spain and says, what Campra has found with micro frame spectroscopy, vibrational bands examinations is not GO, but GH - graphene hydroxid, which is monolayer form from active carbon. And that form of graphene he calls the razor blade, not GO. In next post I shared the link to video on his telegram channel, to both videos, the GO is older. He was talking before a lot about what's going on and PTBs agenda. Also about GO and other s... in jabs, but that last discovery cost him his live and that was also the reason I made this statement about giving up. I can not imagine, how his pregnant girl friend feels. Prayed a lot for both today.
Last edited:
bjorb
The Living Force
They are bluffing. Same with Austria. They can't win when all Hell breaks loose. And they know that.
Natalija8
Jedi
Indian BAR association has charged two billionairs, one is Bill Gates and the other one is Adar Poonawalla, the CEO of SII (Serum Institute of India), the worlds largest vaccine maker, which beside Covishield (Astra Zeneca jab), also produces more than 50% of all vaccines, that are injected into babies.
If convicted, they face the death penalty.



 www.frontnieuws.com
www.frontnieuws.com
EDIT: Don't know why the link didn't copy in English, but on top you can change language settings, there are many languages to choose.
If convicted, they face the death penalty.

Bill Gates in India's Hooggerechtshof aangeklaagd voor COVID-19 vaccinmoord - doodstraf geëist - Frontnieuws
De Indiase Orde van Advocaten meldt dat bij het Indiase Hooggerechtshof een aanklacht wegens moord is ingediend tegen twee miljardairs die verantwoordelijk zijn voor AstraZeneca’s COVID-19 vaccin Covishield, wegens de moord op een 23-jarige man die met het serum werd ingespoten, bericht Brian...
 www.frontnieuws.com
www.frontnieuws.com
EDIT: Don't know why the link didn't copy in English, but on top you can change language settings, there are many languages to choose.
The channel had another video:I just wanted to translate the video in english for our forum and in shock found the video from his pregnani girl friend about him being attacked, shortly after posting his video on 23.11. and that he didn't survive. Her statment is fron saturday, 27.11.
Covid Test für Kinder.. WAS IST DA DRAUF/DRINN ?? Untersuchungen zeigen erschreckendes !!! In English it means: "Covid test for children .. WHAT'S ON IT / INSIDE? Investigations show terrible things!" A German couple had sent sample Covid test sticks for testing in Switzerland. The sticks were of the kind that children are asked to put into their nose to check if their Covid health allows them to go to school. The test came back and showed the presence/traces of two compounds that are poisonous.
From my point of view, that is not surprising, since they should be sterile, but are the traces necessary? Or could they be reduced? We are surrounded by chemical all around us. Laura speaks about it in the intro video to EE. If one looks up the ingredients of household cleaning chemicals, cosmetics, paints, glues, plastics, garden sprays, additives, household insecticides, car fuels, as done next with the two traces compounds in the nose probe sticks, then one might end up being surprised.
What the German couple will work to find out is, if it is safe for children to do the tests daily, for a long time, as is happening now, considering the presence of the following two compounds, ethylene oxide and 2-chloroethanol:
Ethylene oxide
The small print says:
Ethylene Oxide is colorless, odorless, flammable, toxic gaseous cyclic ether with a sweet ether-like smell. Ethylene oxide is used especially in the synthesis of ethylene glycol and as a sterilizing agent for medical supplies and foods, as a fumigant and as an insecticide. Exposure to this substance is highly irritating to the eyes, skin and respiratory tract, induces nausea and vomiting and causes central nervous system depression. Ethylene oxide is mutagenic in humans and chronic exposure is associated with an increased risk of leukemia, stomach cancer, pancreatic cancer and non-Hodgkin lymphoma. (NCI05)
NCI Thesaurus (NCIt)
2-Chloroethanol
Ethylene Chlorohydrin is an organochlorine compound and hazardous substance. Ethylene chlorohydrin is used as a solvent and in the manufacture of a variety of industrial agents.
NCI Thesaurus (NCIt)
Earlier this year, I reported discomfort lasting for some time after being subjected to a Covid nose probe testing in Zürich airport. Eventually I attributed it to a disturbed nerve and symptomatic of latent tension in my right side, but was it caused not only by the mechanical contact, but also by traces of chemicals? One has to be prepared for quite a lot these days.
Coming back to the children who need to test daily to go to school, one could show them how to do the stick test with the least contact and discomfort, just enough to pass. I'm not sure, I would scare them with the presence of the chemical traces. What good would come out of it?
Trending content
-
-
Thread 'Coronavirus Pandemic: Apocalypse Now! Or exaggerated scare story?'
- wanderingthomas
Replies: 30K -
