Federal spending on COVID-19 vaccine candidates tops $9 billion, spread among 7 companies
The federal government has allocated more than $9 billion to develop and manufacture candidate vaccines. More than $2.5 billion more has been earmarked for vials to store the vaccines, syringes to deliver them, and on efforts to ramp up manufacturing and capacity.
And they're not done yet.
So far, the largest sums have gone to pharmaceutical giants Pfizer, AstraZeneca, and a collaboration between Sanofi and GSK, as well as biotech firms Moderna and Novavax – all of which have candidate vaccines being tested in people.
To save time in the development process, the companies have been running trials simultaneously that they usually run in sequence.
Moderna, for instance, hasn't yet published its phase 2 trial results, but is already in larger-scale phase 3 trials, beginning tests last week of its candidate vaccine in 15,000 volunteers. Phase 3 trials started this summer are expected to return results this fall, with the timing depending on how quickly they can find volunteers.
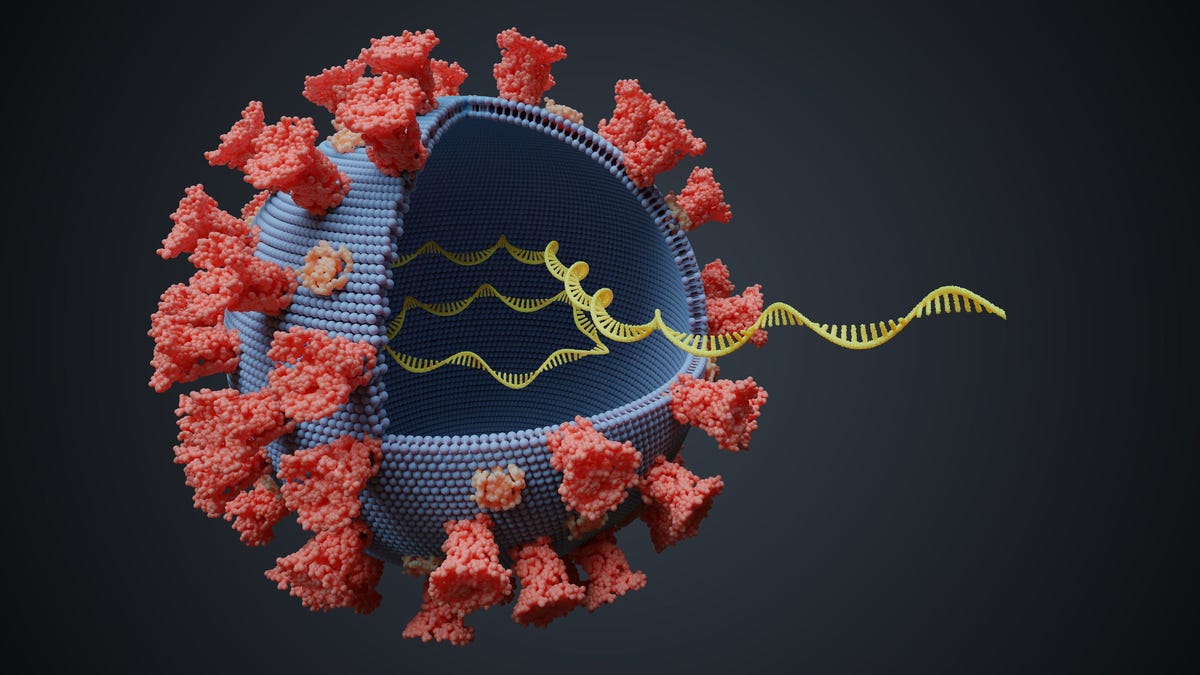
None of the candidate vaccines use the whole virus, so they cannot cause COVID-19. Instead, they train the immune system to respond to the virus' spike protein – which gives the coronavirus its distinctive shape. Once the immune system is trained to recognize the spike protein, it should be able to rapidly clear the virus should the person be exposed again.
The vaccine candidates now receiving government funding are all based on
new technologies,
most of which have not been the basis of previously approved vaccines. They have been chosen because they were faster to develop than more conventional vaccines, which is important in fighting a virus currently killing about 1,000 Americans a day.
If any of these approaches prove safe and effective, it could transform vaccine development worldwide, allowing faster attack strategies against dangerous viruses that may emerge in the future, as well as those that mutate rapidly, like the flu.
Almost all the candidate vaccines in human trials will require two doses to become fully effective, which means many hundreds of millions of doses will be needed to vaccinate the majority of America's 328 million residents.
[...]
Here's
how more than $9 billion taxpayer dollars have been allocated so far for vaccine development:
- Sanofi and GSK: $2.1 billion
- Pfizer and BioNTech: $1.95 billion
- Novavax: $1.6 billion
- Janssen: $1.5 billion
- AstraZeneca and Oxford: $1.2 billion
- Moderna: $955 million
- Merck and IAVI USA: $38 million
Dr. Roger Perlmutter, president of Merck Research Laboratories, said in last week's
earnings report Merck's vaccine will provide protection with only a single dose, and it will be delivered orally, like the polio vaccine, rather than via a shot.
Details:
Amid the coronavirus pandemic, the federal government has allocated more than $9 billion among 7 companies to develop and make COVID-19 vaccine.

www.usatoday.com





 . This a big diffetence compared to Western authorities.
. This a big diffetence compared to Western authorities.