Physicians for Informed Consent Letter Opposing UC Regents’ Flu Vaccine Mandate
September 22, 2020
Michael V. Drake, M.D.
President, University of California Board of Regents,
president@ucop.edu
Cc: Vice President for Human Resources, Executive Vice President for UC Health, University of California Regents Office,
regentsoffice@ucop.edu
Anne Shaw, Secretary and Chief of Staff to the Regents,
anne.shaw@ucop.edu
RE: University of California Executive Order July 31, 2020 (flu vaccine mandate)
Dear Professor Drake,
On behalf of hundreds of physician and scientist members of Physicians for Informed Consent, I am writing out of our concern that the bodily integrity of UC students, faculty, and staff is being potentially sacrificed by the recent UC Regents’ flu vaccine mandate,1 with no robust scientific justification. The data currently available shows the following:
1. People who receive the flu vaccine are 65% more likely to contract non-flu viruses and bacteria than people who do not receive the flu vaccine.
Patients have reported becoming ill following flu vaccination. To address the concern among patients that the flu vaccine causes illness (i.e., acute respiratory illness), the Centers for Disease Control and Prevention (CDC) conducted a three-year study, published in
Vaccine in 2017, to analyze the risk of illness during a time period after flu vaccination compared to the risk of illness in unvaccinated individuals during the same time period.2 The study found there is a 65% increased risk of suffering from a non-flu acute respiratory illness within 14 days of receiving the flu vaccine. The authors state, “Patients’ experiences of illness after vaccination may be validated by these results.”
This is important because although flu vaccines typically target at most four strains of flu virus,3 over 200 different viruses cause illnesses that produce the same symptoms—fever, headache, aches, pains, cough, and runny nose—as influenza,4 and more than 85% of acute respiratory illnesses do not involve the flu.
2. There is evidence that the flu vaccine doesn’t reduce demand on hospitals.
The studies referenced in the UC Regents’ flu vaccine mandate suggest positive effects of the flu vaccine on the incidence of illness caused by flu viruses; however, that benefit may be outweighed by the negative effects of the flu vaccine on the incidence of non-flu respiratory illness. A 2018 Cochrane review of 52 clinical trials assessing the effectiveness of influenza vaccines did not find a significant difference in hospitalizations between vaccinated and unvaccinated adults. Instead, the reviewers found “low-certainty evidence that hospitalization rates and time off work may be comparable between vaccinated and unvaccinated adults.”6
Furthermore, a Mayo Clinic study published in 2012 found “a threefold increased risk of hospitalization in subjects who did get the TIV [trivalent inactivated influenza] vaccine.”7
3. There is no evidence that the flu vaccine prevents the spread of influenza viruses.
Households are thought to play a major role in community spread of influenza, and there has been a long history of analyzing family households to study the incidence and transmission of respiratory illnesses of all severities. As such, the CDC funded a study of 1,441 participants, both vaccinated and unvaccinated, in 328 households. The study, published in
Clinical Infectious Diseases, evaluated the flu vaccine’s ability to prevent community-acquired influenza (household index cases) and influenza acquired in people with confirmed household exposure to the flu (secondary cases). Transmission risks were determined and characterized. In conclusion, the authors state: “There was no evidence that vaccination prevented household transmission once influenza was introduced.”8,9
Furthermore, a systematic review of 50 influenza vaccine studies conducted for the Cochrane Library states: “Influenza vaccines have a modest effect in reducing influenza symptoms and working days lost. There is no evidence that they affect complications, such as pneumonia, or transmission.”5
4. The flu vaccine has not reduced pneumonia and influenza mortality.
The National Vaccine Program Office, a division of the U.S. Department of Health and Human Services (HHS), funded a study to examine flu mortality over the period of 33 years (1968–2001). The study found that there has been no decrease in flu mortality since the widespread use of the influenza vaccine. The authors state: “We could not correlate increasing vaccination coverage after 1980 with declining mortality rates in any age group… [W]e conclude that observational studies substantially overestimate vaccination benefit.”10
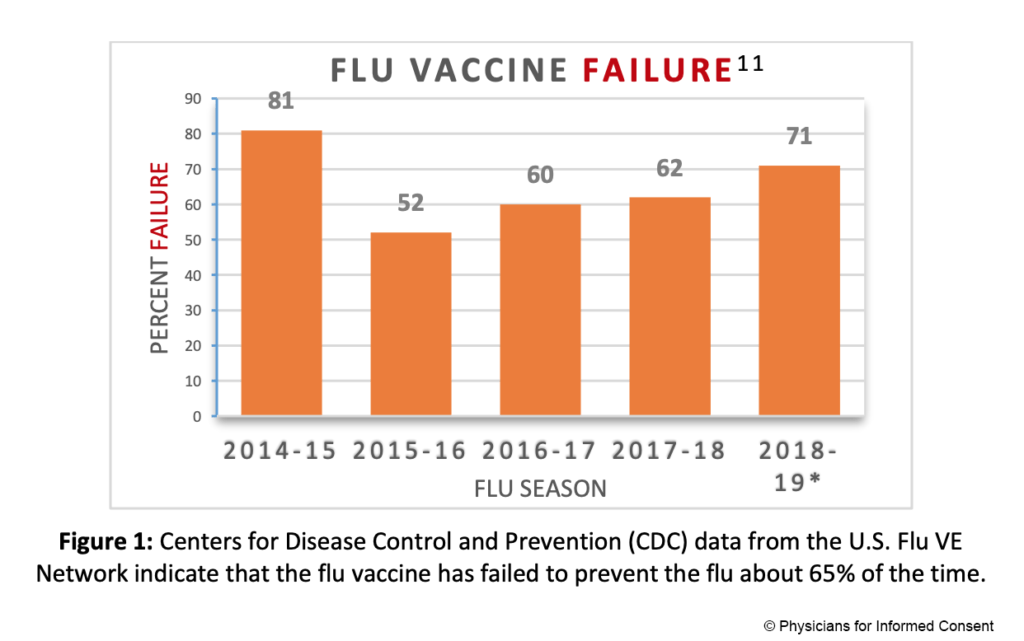
5. The flu vaccine fails to prevent the flu about 65% of the time.
The CDC conducts studies to assess the effects of flu vaccination each flu season to help determine if flu vaccines are working as intended.11,12 As the flu viruses that are circulating are constantly changing (primarily due to antigenic drift mutations),13 flu vaccines are reformulated regularly based on a “best guess” of which viruses might circulate during the coming flu season.14 The CDC states: “CDC monitors vaccine effectiveness annually through the Influenza Vaccine Effectiveness (VE) Network, a collaboration with participating institutions in five geographic locations… [A]nnual estimates of vaccine effectiveness give a real-world look at how well the vaccine protects against influenza caused by circulating viruses each season.”12
Data from the CDC’s Influenza VE Network indicate a 65% vaccine failure rate between 2014 and 2018 (Fig. 1).11
6. Repeat flu vaccination has been shown to increase the likelihood of flu vaccine failure.
Studies have observed that influenza vaccines have a high failure rate in individuals who are vaccinated in two consecutive years.8 A review of 17 influenza vaccine studies published in
Expert Review of Vaccines states, “The effects of repeated annual vaccination on individual long-term protection, population immunity, and virus evolution remain largely unknown.”15
7. The overall benefits of flu vaccination and flu vaccine policies are not clear.
A Cochrane Vaccines Field analysis evaluated studies measuring the benefits of flu vaccination. The analysis, published in the
BMJ, concludes: “The large gap between policy and what the data tell us (when rigorously assembled and evaluated) is surprising… Evidence from systematic reviews shows that inactivated vaccines have little or no effect on the effects measured… Reasons for the current gap between policy and evidence are unclear, but given the huge resources involved, a re-evaluation should be urgently undertaken.”
Finally, it’s important to remember that since the enactment of the National Childhood Vaccine Injury Act of 1986,17 which has shielded both vaccine manufacturers and physicians from vaccine injury lawsuits, the National Vaccine Injury Compensation Program has awarded over $4 billion to people who incurred vaccine injuries and deaths.18 These individuals and their families have a heightened awareness of their risk of vaccine injury, whether or not their injuries fall under the CDC list of contraindications or precautions; and flu vaccine injury claims are the most common.
We urge you to rescind the UC Regents’ flu vaccine mandate as it thwarts the ability of your students, faculty, and staff to exercise their ability to refuse a medical procedure. There is no medical justification for requiring people to potentially sacrifice their bodily integrity and health in order to work or obtain an education.
Respectfully,
Shira Miller, M.D.
Founder and President
Physicians for Informed Consent
Physicians for Informed Consent (PIC) is a 501(c)(3) nonprofit educational organization that delivers data on infectious diseases and vaccines, and unites doctors, scientists, healthcare professionals, attorneys, and families who support voluntary vaccination. Its Coalition for Informed Consent (CIC) includes over 200 member organizations.
Download/Print PDF
References
- University of California. Regents of the University of California. University of California executive order July 31, 2020; [cited 2020 Aug 17]. https://ucnet.universityofcalifornia.edu/news/2020/08/2020-21-flu-vaccination-executive-order.pdf.
- Rikin S, Jia H, Vargas CY, Castellanos de Belliard Y, Reed C, LaRussa P, Larson EL, Saiman L, Stockwell MS. Assessment of temporally related acute respiratory illness following influenza vaccination. Vaccine. 2018 Apr 5;36(15):1958-64.
- Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. Table 1: influenza vaccines—United States, 2020–21 influenza season; [cited 2020 Sep 3]. TABLE 1. Influenza vaccines — United States, 2020–21 influenza season* | CDC.
- Demicheli V, Jefferson T, Al-Ansary LA, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database of Syst Rev. 2014 Mar 13;(3):CD001269.
- Jefferson T, Di Pietrantonj C, Rivetti A, Bawazeer GA, Al-Ansary LA, Ferroni E. Vaccines for preventing influenza in healthy adults. Cochrane Database Sys Rev. 2010 Jul 7;(7):CD001269.
- Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD001269.
- Joshi AY, Iyer VN, Hartz MF, Patel AM, Li JT. Effectiveness of trivalent inactivated influenza vaccine in influenza-related hospitalization in children: a case-control study. Allergy Asthma Proc. 2012 Mar-Apr;33(2):e23-7.
- Ohmit SE, Petrie JG, Malosh RE, Cowling BJ, Thompson MG, Shay DK, Monto AS. Influenza vaccine effectiveness in the community and the household. Clin Infect Dis. 2013 May;56(10):1363.
- Physicians for Informed Consent. Newport Beach (CA): Physicians for Informed Consent. Vaccines: what about immunocompromised schoolchildren? Dec 2019. Immunocompromised Schoolchildren - Risk Group Information Statement (RGIS) — Physicians for Informed Consent.
- Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med. 2005 Feb 14;165(3):265-72.
- Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. CDC seasonal flu vaccine effectiveness studies; [cited 2020 Apr 17]. CDC Seasonal Flu Vaccine Effectiveness Studies | CDC.
- Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. How flu vaccine effectiveness and efficacy are measured; [cited 2020 May 14]. How Flu Vaccine Effectiveness and Efficacy are Measured | CDC.
- Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. Influenza (flu): how flu viruses can change; [cited 2020 Aug 17]. How Flu Viruses Can Change.
- Centers for Disease Control and Prevention. Washington, D.C.: U.S. Department of Health and Human Services. Influenza (flu): selecting viruses for the seasonal influenza vaccine; [cited 2020 Aug 17]. Selecting Viruses for the Seasonal Flu Vaccine.
- Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines. 2017 Jul;16(7):723,733.
- Jefferson T. Influenza vaccination: policy versus evidence. BMJ. 2006 Oct 28;333(7574):912-5.
- Congress.gov. Washington, D.C.: Library of Congress (LOC). H.R.5546 – National Childhood Vaccine Injury Act of 1986; [cited 2020 Aug 17]. H.R.5546 - 99th Congress (1985-1986): National Childhood Vaccine Injury Act of 1986.
- National Vaccine Injury Compensation Program. Rockville (MD): Health Resources and Services Administration. National Vaccine Injury Compensation Program: monthly statistics report; [updated 2019 Jun 1; cited 2020 Aug 17]. https://www.hrsa.gov/sites/default/files/hrsa/vaccine-compensation/data/monthly-stats-june-2019.pdf.
*Estimates presented to the Advisory Committee on Immunization Practices on June 27, 2019

 childrenshealthdefense.org
childrenshealthdefense.org
 www.sciencemag.org
www.sciencemag.org